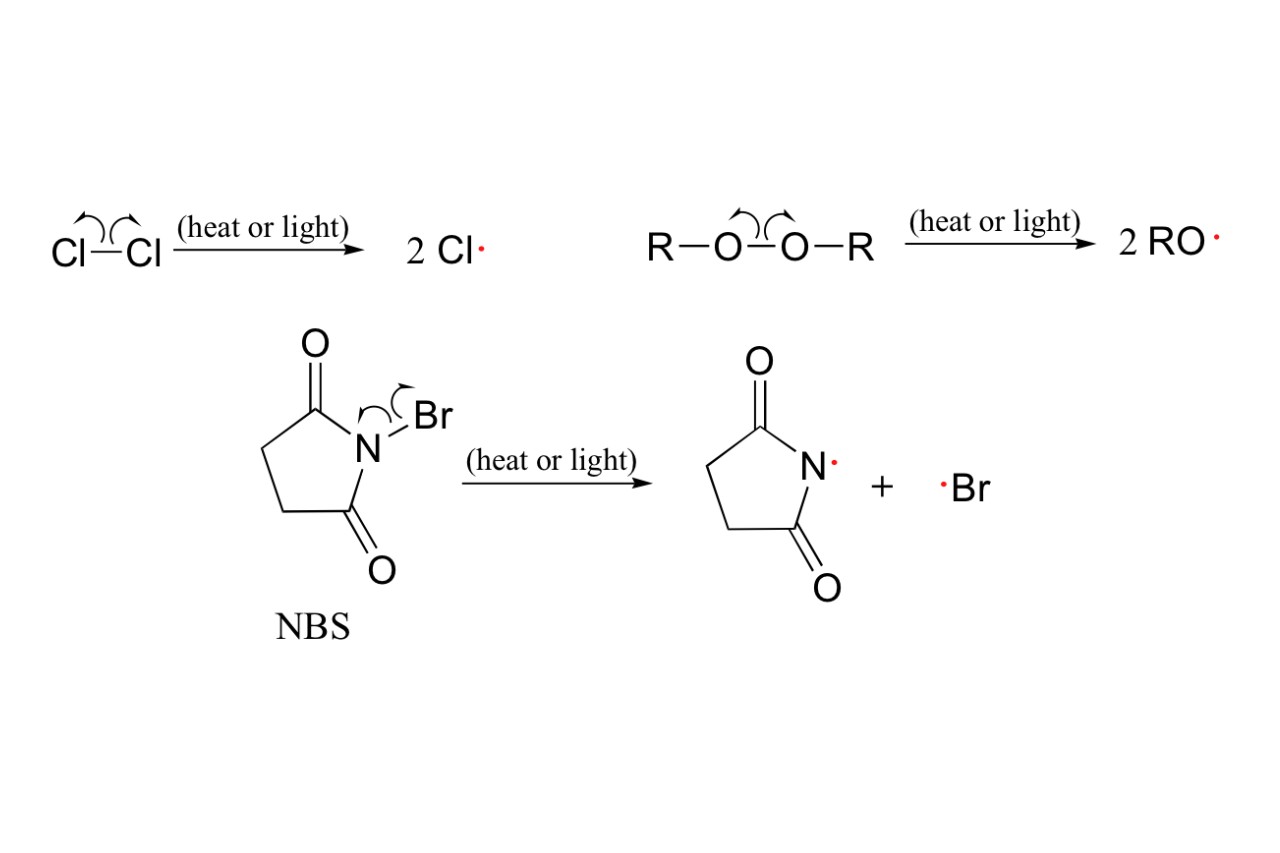
Radical reactions are at the heart of organic chemistry, playing a crucial role in a wide range of chemical processes. These reactions involve the formation and rearrangement of highly reactive species called radicals, which possess an unpaired electron. While radicals may seem enigmatic, they have fascinated chemists for decades and continue to be an area of intense research and exploration.
In this article, we will delve into the world of radical reactions and uncover eight intriguing facts that will enhance your understanding of this fundamental chemical phenomenon. From the role of radicals in natural processes to their applications in synthetic chemistry, get ready to discover the fascinating world of radical reactions. So, let’s dive in and explore the captivating world of radicals!
Key Takeaways:
- Radical reactions are like the superheroes of chemistry, creating highly reactive species called radicals. They’re rapid, unpredictable, and essential for making things like plastics and studying the air we breathe.
- Radical reactions aren’t just in test tubes; they also happen in our bodies and the air around us. They can be helpful, like in making new molecules, but also harmful, causing damage to our cells and the environment.
What is Radical Reaction?
Radical reaction is a type of chemical reaction that involves the formation and subsequent reactivity of radicals, which are highly reactive species due to the presence of unpaired electrons. These reactions play a crucial role in organic synthesis, polymerization processes, and atmospheric chemistry.
Rapid and Unpredictable!
One intriguing aspect of radical reactions is their rapid and sometimes unpredictable nature. Unlike other types of reactions, radical reactions occur via a chain mechanism, where reactive intermediates called radicals are continuously regenerated.
Radical Clocks
Ever heard of a clock that measures reaction rates? Well, in radical chemistry, such clocks exist! Known as “radical clocks,” these compounds undergo predictable reactions at specific time intervals when exposed to radicals, allowing scientists to study reaction kinetics.
Radical Polymerization
Radical reactions are crucial in the synthesis of polymers. In radical polymerization, monomers containing double bonds undergo a chain reaction, resulting in the formation of long chains of repeating units.
Initiation, Propagation, and Termination
Radical reactions occur in three main steps: initiation, propagation, and termination. During initiation, radicals are formed, often through the use of initiators like peroxides. In propagation, radicals react with monomers, causing a chain reaction. Finally, termination occurs when radicals combine or react with another molecule to form stable products.
Radical Substitution Reactions
Radical substitution reactions are a common type of radical reaction. One well-known example is the halogenation of alkanes, where a hydrogen atom is replaced by a halogen atom due to the reactivity of alkyl radicals.
Radical Reactions in Atmospheric Chemistry
Radical reactions play a vital role in atmospheric chemistry. For example, the reaction of hydroxyl radicals (·OH) with pollutants like nitrogen oxides (NOx) and volatile organic compounds (VOCs) leads to the formation of smog and ozone, affecting air quality and human health.
Radical Reactions in Biological Systems
Radical reactions are not limited to the lab or industrial processes. They also occur within living organisms. Radicals, such as reactive oxygen species, are generated during various metabolic processes and can cause oxidative damage to DNA, proteins, and lipids, leading to aging, disease, and cell death.
Conclusion
In conclusion, radical reactions are a fascinating aspect of chemistry that play a pivotal role in various chemical processes. They involve the formation and subsequent reactivity of highly reactive species called radicals. Understanding the principles and mechanisms behind radical reactions is crucial for advancements in pharmaceuticals, materials science, and environmental science.These 8 intriguing facts about radical reactions shed light on their importance and complexity. From their role in organic synthesis to their involvement in atmospheric chemistry, radical reactions have a significant impact on our daily lives. By harnessing the power of radicals, scientists can develop new methods, accelerate chemical reactions, and discover innovative solutions to pressing challenges.As we continue to explore the world of chemistry, radical reactions will undoubtedly remain a captivating field of study. Their unique nature and wide range of applications make them an area of ongoing research and discovery. By delving deeper into the mechanisms and intricacies of radical reactions, we can unlock new opportunities and push the boundaries of scientific understanding.
FAQs
Q: What is a radical reaction?
A radical reaction is a type of chemical reaction that involves the formation and reactivity of highly reactive species known as radicals. These radicals have unpaired electrons and are extremely reactive, leading to various types of reactions.
Q: How do radical reactions occur?
Radical reactions typically occur through a process known as homolysis, where a bond is cleaved, and each atom receives one of the shared electrons. This results in the formation of two radicals, which can then participate in further reactions.
Q: What is the significance of radical reactions in organic synthesis?
Radical reactions provide unique and powerful tools for organic synthesis. They enable the formation of complex organic molecules by facilitating the construction of carbon-carbon and carbon-heteroatom bonds that are otherwise challenging to create.
Q: Are radical reactions only applicable to organic chemistry?
No, radical reactions are not limited to organic chemistry. They also play a crucial role in areas such as polymer chemistry, atmospheric chemistry, and biochemistry.
Q: Can radical reactions be controlled?
Controlling radical reactions can be challenging due to their highly reactive nature. However, advances in catalysis and reaction conditions have enabled better control over radical reactions, allowing scientists to achieve specific outcomes.
Q: What are the environmental implications of radical reactions?
Radical reactions in the atmosphere are responsible for the formation and degradation of pollutants such as ozone and smog. Understanding these reactions is essential in mitigating air pollution and developing strategies for environmental sustainability.
Q: How are radical reactions used in industry?
Radical reactions find applications in various industrial processes, such as polymerization, drug synthesis, and the production of specialty chemicals. They offer efficient and economical routes to generate desired products on a large scale.
Q: What are future directions in research on radical reactions?
Future research on radical reactions aims to explore new catalysts and reaction conditions, develop more selective methodologies, and uncover novel radical species. This ongoing research will continue to expand our understanding and applications of radical reactions in various fields.
Radical reactions are just the beginning of your chemistry journey! Dive deeper into the world of chemical processes and uncover more fascinating facts. Explore the intricacies of polymerization and learn how small molecules combine to form large, complex structures. Gain a deeper understanding of reaction mechanisms, the step-by-step processes behind chemical transformations. Don't forget about the importance of free radicals and their impact on our health – discover the role of antioxidants in combating these reactive species. Get ready to expand your chemistry knowledge with these captivating topics!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
