Reaction coordinate is a fundamental concept in chemistry that plays a crucial role in understanding chemical reactions. It refers to the path taken by a reaction as it progresses from reactants to products. The reaction coordinate provides insight into the energy changes and structural rearrangements that occur during the reaction.
In this article, we will uncover 14 captivating facts about reaction coordinate that will enhance your understanding of this intriguing concept. From the role of transition states to the effect of catalysts, these facts will shed light on the intricacies and nuances of chemical reactions.
Whether you are a chemistry enthusiast or a student studying the subject, these fascinating facts will deepen your appreciation for the complexity and beauty of chemical reactions. So, let’s dive in and explore the captivating world of reaction coordinate!
Key Takeaways:
- Reaction coordinate shows the path of a chemical reaction and helps scientists understand how energy changes during the reaction. It’s like a map for understanding how reactions happen!
- Reaction coordinate diagrams help scientists figure out how fast reactions occur and what factors affect them. It’s like a secret code that unlocks the mysteries of chemical reactions!
What is Reaction Coordinate?
Reaction coordinate refers to the path or trajectory followed by a chemical reaction from the reactants to the products. It provides a visual representation of the energy changes that occur during the course of a reaction.
The Role of Reaction Coordinate
The reaction coordinate helps in understanding the progress and mechanism of a chemical reaction. It allows scientists to determine key factors such as activation energy, reaction rate, and transition state.
Reaction Coordinate Diagrams
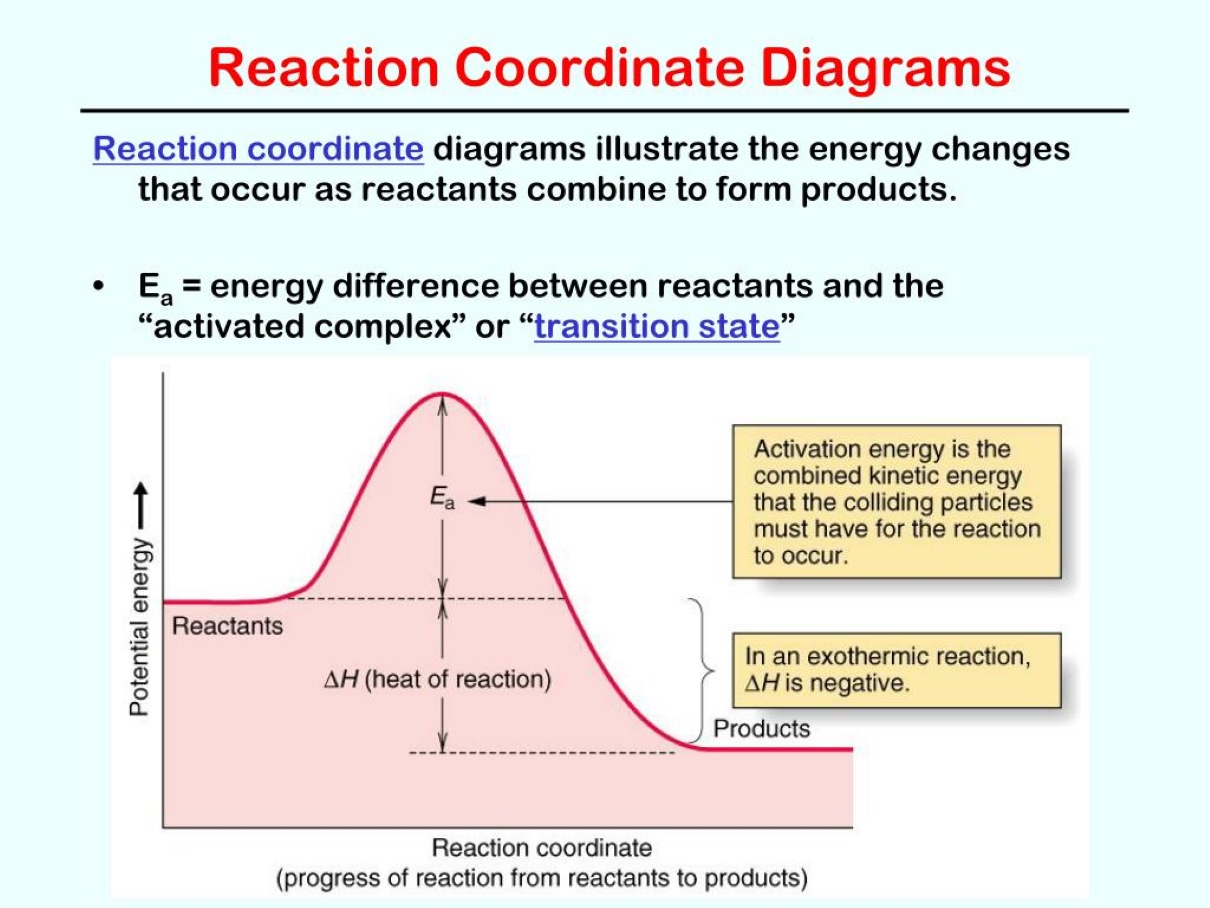
Reaction coordinate diagrams illustrate the energy changes during a chemical reaction. They show the energy of the reactants, products, and intermediates along the reaction pathway.
Transition State
The transition state is a high-energy state that occurs at the peak of the reaction coordinate diagram. It represents the moment when the reactants are transforming into products.
Activation Energy
Activation energy is the energy barrier that must be overcome for a reaction to occur. It is the difference in energy between the reactants and the transition state.
Reaction Coordinate and Equilibrium
The reaction coordinate helps determine the position of equilibrium in a chemical reaction. It allows scientists to study the overall energy changes and determine the stability of the system.
Reaction Coordinate and Catalysts
Catalysts lower the activation energy of a reaction, providing an alternative reaction coordinate with a lower energy barrier. This allows the reaction to occur more quickly and efficiently.
Reaction Coordinate and Rate of Reaction
The reaction coordinate provides insights into the rate of a chemical reaction. By studying the energy changes along the pathway, scientists can determine the speed at which the reaction proceeds.
Reaction Coordinate and Reaction Mechanisms
Reaction mechanisms describe the step-by-step sequence of elementary reactions that lead to the overall reaction. The reaction coordinate helps in understanding and predicting these mechanisms.
Reaction Coordinate and Activation Complex
Activation complexes are short-lived species that form at the transition state of a chemical reaction. They play a crucial role in determining the reaction pathway and product formation.
Reaction Coordinate and Reaction Coordinate Diagrams
Reaction coordinate diagrams are graphical representations of the energy changes that occur during a chemical reaction. They help visualize the progress and energetics of the reaction.
Reaction Coordinate and Gibbs Free Energy
Reaction coordinate allows for the calculation of Gibbs free energy, a measure of the maximum useful work that can be extracted from a reaction. It helps in determining the spontaneity and feasibility of a reaction.
Reaction Coordinate and Reaction Pathway
The reaction coordinate depicts the reaction pathway, which is the specific sequence of steps that a reaction follows from reactants to products. It helps provide insights into the intermediates and intermediacy steps involved.
Reaction Coordinate and Reaction Kinetics
The reaction coordinate is closely linked to reaction kinetics, the study of reaction rates. It allows scientists to analyze the factors that affect the rate of a reaction, such as temperature, concentration, and catalysts.
Conclusion
In conclusion, understanding the concept of reaction coordinate is crucial in the field of chemistry. It provides a visualization of the energy changes that occur during a chemical reaction, allowing scientists to analyze and predict reaction outcomes. By studying the reaction coordinate, chemists can determine the activation energy, intermediates, and transition states involved in a reaction.The reaction coordinate also helps in understanding the factors that influence the rate of a reaction. By manipulating the reaction conditions and studying the corresponding changes in the reaction coordinate, scientists can gain insights into the reaction mechanism and optimize the reaction conditions for desired outcomes.Overall, the reaction coordinate offers a valuable tool for chemists to investigate and comprehend the intricacies of chemical reactions. Its applications extend to various fields, including pharmaceuticals, materials science, and environmental research, making it an essential concept for anyone involved in the study of chemistry.
FAQs
1. What is a reaction coordinate?
A reaction coordinate is a representation of the energy changes that occur during a chemical reaction. It shows the progression of reactants to products along a specific pathway.
2. How is the reaction coordinate determined?
The reaction coordinate is determined by taking into account the changes in energy as the reaction progresses. This can be done by analyzing the potential energy surface and identifying the transition states and intermediates.
3. Why is the reaction coordinate important?
The reaction coordinate is important because it provides valuable insights into the reaction mechanism, activation energy, and intermediates involved in a chemical reaction. It helps in understanding the factors that influence the rate and outcome of a reaction.
4. Can the reaction coordinate be altered?
The reaction coordinate can be altered by changing the reaction conditions such as temperature, pressure, or the presence of catalysts. These changes can affect the energy barriers and intermediates along the reaction pathway.
5. What are some practical applications of studying the reaction coordinate?
Studying the reaction coordinate is essential in fields such as drug development, materials science, and environmental research. It helps in designing efficient synthetic routes, understanding catalysts, and predicting reaction outcomes.
Reaction coordinate fascinates chemists, providing insights into chemical reactions' progress. Delving deeper into this concept reveals its significance in determining reaction rates, energy requirements, and mechanisms. Understanding reaction coordinate is essential for those interested in chemical kinetics, as it directly relates to the activation energy needed for reactions to occur. Moreover, reaction coordinate plays a crucial role in catalysis, where catalysts lower activation energy barriers, accelerating reactions. Exploring reaction coordinate opens doors to a deeper understanding of chemical processes and their optimization.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
