When it comes to the fascinating world of chemistry, Lewis bases are a topic that never fails to intrigue. These compounds, named after famous chemist Gilbert N. Lewis, play a crucial role in numerous chemical reactions and have a profound impact on our everyday lives. Understanding the properties and behavior of Lewis bases is essential for comprehending a wide range of chemical phenomena, from acid-base reactions to coordination chemistry.
In this article, we will delve into the captivating world of Lewis bases and explore 12 mind-blowing facts that will surely leave you in awe. Whether you are a chemistry enthusiast, a student, or simply someone curious about the wonders of the chemical realm, brace yourself for an exciting journey as we unravel the secrets of these remarkable compounds.
Key Takeaways:
- Lewis bases are like chemical matchmakers, helping form new compounds in acid-base reactions by donating electron pairs to Lewis acids.
- They play a vital role in chemistry, from forming metal complexes to catalyzing reactions, and are essential in various scientific disciplines.
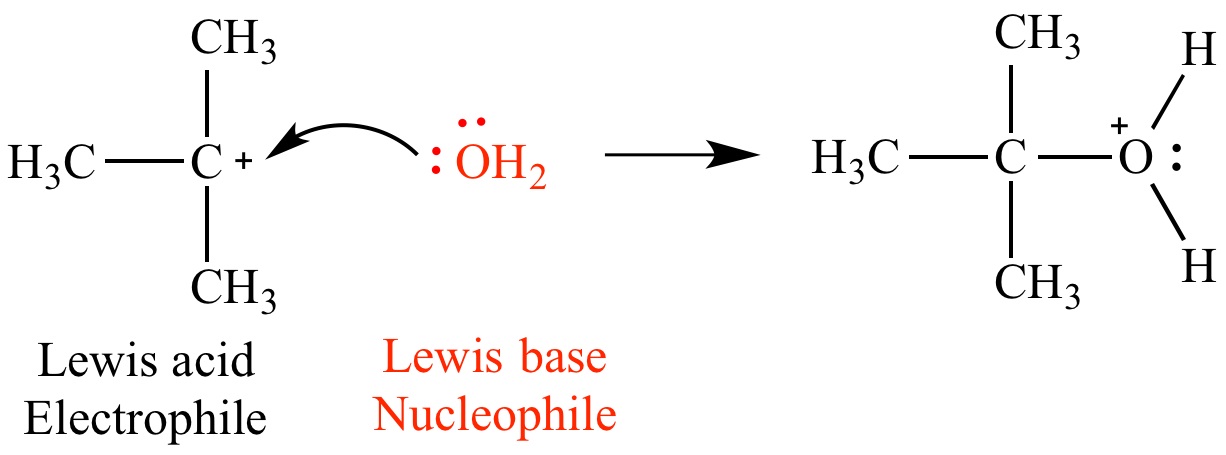
Lewis bases play a crucial role in acid-base reactions.
One of the fundamental principles in chemistry is acid-base reactions. Lewis bases provide the electron pair necessary to form a bond with a Lewis acid, resulting in the formation of a new compound.
Lewis bases can be classified as hard or soft.
Based on their electrostatic properties, Lewis bases can be categorized as hard or soft. Hard bases have a stronger affinity for hard acids, while soft bases prefer to interact with soft acids.
Lewis bases are commonly used in coordination chemistry.
Coordination compounds, which consist of a central metal ion surrounded by ligands, heavily rely on Lewis bases to donate electron pairs and form coordination bonds.
Lewis bases can act as ligands in metal complexes.
Many Lewis bases have the ability to form coordinate bonds with metal ions and form stable metal complexes. These complexes often exhibit unique chemical and physical properties.
Lewis bases can participate in Lewis acid-base adduct formation.
When a Lewis base donates an electron pair to a Lewis acid, an adduct is formed. This adduct is a temporary species that is crucial in many chemical reactions.
Lewis bases are essential in Lewis acid-catalyzed reactions.
Several chemical reactions, especially those involving polar reagents or substrates, require the presence of Lewis acids. Lewis bases play a vital role in these reactions by interacting with the Lewis acid catalyst and stabilizing the transition state.
Lewis bases can modify the reactivity of organic molecules.
By interacting with organic molecules, Lewis bases can alter their electrostatic environment and influence their reactivity in various chemical transformations.
Lewis bases are frequently used in organic synthesis.
Organic chemists rely on Lewis bases to facilitate reactions such as nucleophilic substitution, addition, and elimination, which are crucial in synthesizing complex organic compounds.
Lewis bases are involved in the formation of Lewis acid-base complexes.
When a Lewis base and a Lewis acid interact, they form a Lewis acid-base complex. These complexes can range from simple to highly complex structures depending on the nature of the components involved.
Lewis bases can act as catalysts in certain chemical reactions.
Some Lewis bases are capable of activating certain substrates or reagents, thereby catalyzing specific chemical reactions.
Lewis bases can participate in hydrogen bonding.
In addition to forming coordinate bonds with Lewis acids, Lewis bases can also engage in hydrogen bonding interactions with hydrogen-containing compounds.
Lewis bases are widely studied and utilized in various scientific disciplines.
Due to their fundamental role in chemical reactions, Lewis bases have garnered significant attention from researchers in fields such as medicinal chemistry, materials science, and environmental chemistry.
As you can see, Lewis bases are essential components of many chemical reactions and have diverse applications in different scientific disciplines. Understanding their properties and interactions with Lewis acids allows scientists to better comprehend the complexity of chemical systems. The 12 mind-blowing facts about Lewis bases presented here offer a glimpse into the intriguing world of Lewis bases and highlight their significance in the realm of chemistry.
Conclusion
In conclusion, exploring the fascinating world of Lewis bases reveals an array of mind-blowing facts. From their role in chemical reactions to their significance in understanding molecular structures, Lewis bases are essential in the field of chemistry. Understanding the broad range of Lewis bases and their properties is crucial for chemists and scientists alike. The ability of Lewis bases to donate an electron pair and form coordinated compounds is not only intriguing but also plays a vital role in various applications, including catalysis, pharmaceuticals, and materials science. As we continue to delve deeper into the field of chemistry, the study of Lewis bases will undoubtedly continue to expand, uncovering even more mind-blowing facts and contributing to the advancement of scientific knowledge.
FAQs
Q: What is a Lewis base?
A: A Lewis base is a substance that can donate a pair of electrons and form a coordinate bond with an electron-deficient species, known as a Lewis acid.
Q: What are some examples of Lewis bases?
A: Some examples of Lewis bases include ammonia (NH3), water (H2O), pyridine (C5H5N), and hydroxide ion (OH-).
Q: How do Lewis bases participate in chemical reactions?
A: Lewis bases donate a pair of electrons to a Lewis acid, forming a coordinate bond. This interaction plays a vital role in various chemical reactions, such as acid-base reactions and coordination chemistry.
Q: Are all bases Lewis bases?
A: No, not all bases are Lewis bases. While all Lewis bases are bases, not all bases can form coordinate bonds with Lewis acids. For example, hydroxide ion (OH-) is a Lewis base, but sodium hydroxide (NaOH) is not a Lewis base.
Q: What are the applications of Lewis bases?
A: Lewis bases play a crucial role in many applications. They are used in catalysis to increase the rate of chemical reactions, as ligands in coordination chemistry to form complex compounds, and in the development of pharmaceuticals and materials science, among other fields.
Intrigued by Lewis bases? Dive deeper into chemistry with our captivating articles. Unravel the mysteries of acid-base reactions through Bronsted-Lowry theory. Expand your knowledge with mind-boggling chemistry facts. Explore the fascinating world of coordination compounds and their unique properties. Embark on a journey of discovery as you uncover the secrets behind chemical reactions and bond formations. Don't miss out on these incredible opportunities to enhance your understanding of chemistry and its many applications in our everyday lives.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
