Nucleophilic substitution is a fundamental concept in organic chemistry that plays a crucial role in understanding reactions involving organic compounds. It involves the substitution of an atom or a group in a molecule with a nucleophile, which is an electron-rich species. This process occurs through a series of intricate molecular interactions and greatly impacts the reactivity and behavior of various organic compounds.
In this article, we will explore fifteen captivating facts about nucleophilic substitution that will deepen your understanding of this essential concept in chemistry. From the mechanisms behind nucleophilic substitutions to its relevance in different reactions and applications in organic synthesis, we will take a comprehensive dive into the fascinating world of nucleophilic substitution.
Key Takeaways:
- Nucleophilic substitution is a key reaction in chemistry, where a nucleophile replaces an atom or group in a molecule. It’s like a chemical game of “musical chairs” with atoms and groups swapping places!
- Understanding nucleophilic substitution helps scientists create new drugs, modify molecules, and study biological processes. It’s like having a secret code to unlock the mysteries of chemistry and make amazing discoveries!
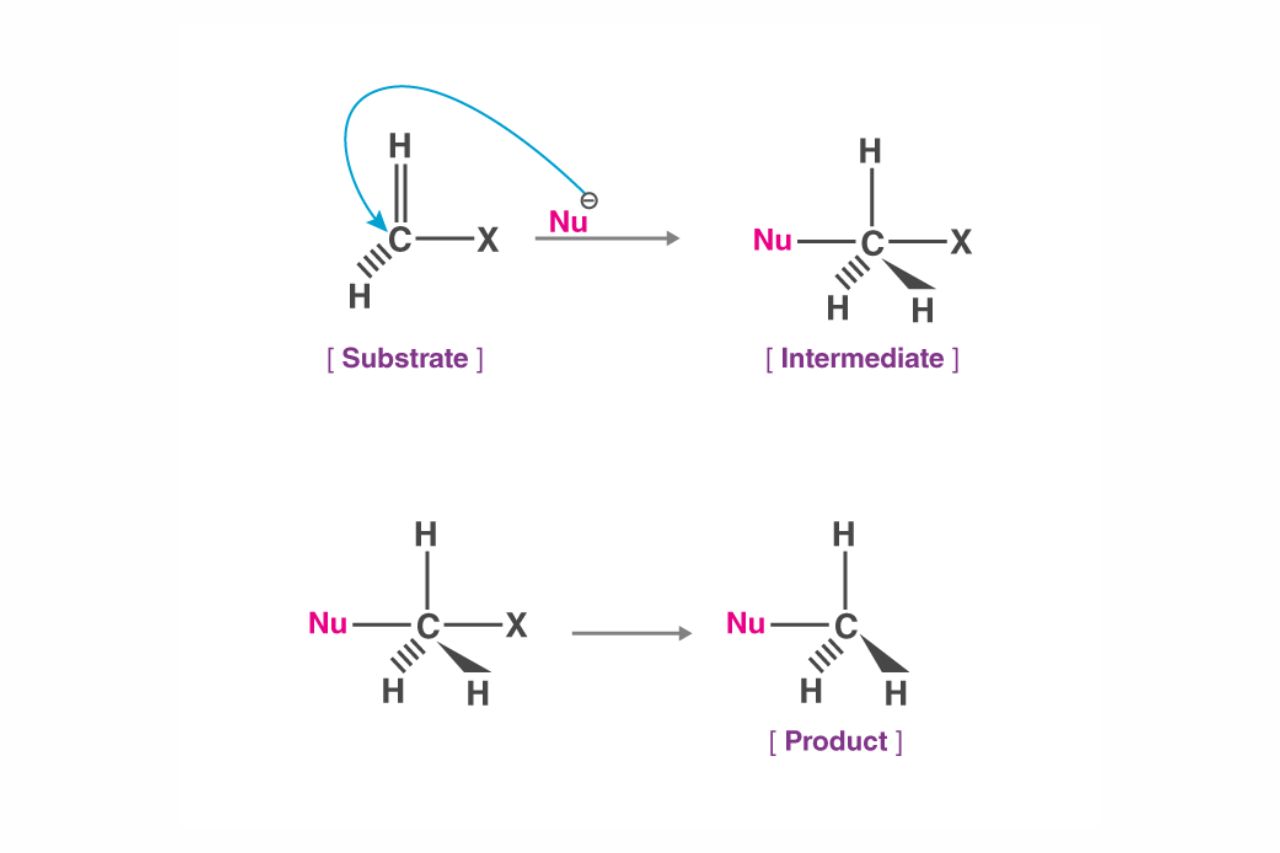
The Mechanism of Nucleophilic Substitution
Nucleophilic substitution is a fundamental reaction in organic chemistry that involves the replacement of an atom or a group in a molecule by a nucleophile. It occurs when a nucleophile attacks an electrophilic center, resulting in the formation of a new bond and the departure of a leaving group. This reaction mechanism plays a crucial role in various organic transformations.
Nucleophiles and Leaving Groups
Nucleophilic substitution reactions require the presence of both a nucleophile and a leaving group. Nucleophiles are electron-rich species that donate a pair of electrons to form a new bond, while leaving groups are atoms or groups that leave the molecule, often taking the electrons with them. The choice of nucleophile and leaving group greatly influences the rate and outcome of the reaction.
Types of Nucleophilic Substitution Reactions
There are two main types of nucleophilic substitution reactions: SN1 (substitution nucleophilic unimolecular) and SN2 (substitution nucleophilic bimolecular). SN1 reactions occur in two steps, involving the formation of a carbocation intermediate, while SN2 reactions proceed in a single step with simultaneous bond formation and bond breaking.
Nucleophilic Substitution in Organic Synthesis
Nucleophilic substitution reactions are widely used in organic synthesis to introduce functional groups, modify molecules, and create complex organic compounds. These reactions offer a versatile tool for chemists to build up molecular complexity and achieve desired molecular structures.
Factors Affecting the Reaction Rate
The rate of nucleophilic substitution reactions can be influenced by several factors. These include the nature of the nucleophile and the leaving group, the steric hindrance around the reaction center, the solvent polarity, and the temperature. Understanding and controlling these factors are key to optimizing the reaction conditions.
Nucleophilic Substitution in Biological Systems
Nucleophilic substitution reactions also play a crucial role in biological systems. They are involved in various physiological processes, such as DNA replication, protein synthesis, and enzymatic catalysis. Understanding these reactions is essential for studying and developing drugs targeting specific biological pathways.
Examples of Nucleophilic Substitution Reactions
There are numerous examples of nucleophilic substitution reactions in organic chemistry. One common example is the reaction between an alkyl halide and a nucleophile, resulting in the substitution of the halide by the nucleophile. Other examples include the hydrolysis of esters, the reaction of Grignard reagents with carbonyl compounds, and the conversion of alcohols to alkyl halides.
The Role of Solvents
The choice of solvent can significantly impact nucleophilic substitution reactions. Polar aprotic solvents, such as acetone and DMF, are commonly used for SN2 reactions, while polar protic solvents, like water and alcohols, are favored for SN1 reactions. The solvent plays a crucial role in solubility, nucleophile stabilization, and ion mobility.
Nucleophilic Substitution versus Elimination Reactions
Nucleophilic substitution reactions often compete with elimination reactions, leading to different products. Elimination reactions result in the removal of a leaving group and the formation of a double bond. Understanding the factors that favor one pathway over the other is essential for controlling the selectivity of the reaction.
Unimolecular Nucleophilic Substitution (SN1) Reactions
SN1 reactions proceed through a two-step mechanism, involving the formation of a carbocation intermediate. The rate-determining step is the dissociation of the leaving group, followed by the attack of the nucleophile. The reaction rate depends only on the concentration of the substrate and is independent of the nucleophile’s concentration.
Bimolecular Nucleophilic Substitution (SN2) Reactions
SN2 reactions occur in a single step, where the nucleophile attacks the electrophilic carbon center while the leaving group departs. This concerted mechanism requires the nucleophile and the leaving group to collide simultaneously. The reaction rate depends on the concentration of both the substrate and the nucleophile.
Organic Reactions Involving Nucleophiles
Nucleophiles participate in various organic reactions beyond nucleophilic substitution. They can also be involved in addition reactions, ring-opening reactions, and nucleophilic addition-elimination reactions. The versatility of nucleophiles allows them to engage in diverse chemical transformations.
Stereoselectivity in Nucleophilic Substitution Reactions
Nucleophilic substitution reactions can exhibit stereoselectivity, leading to the formation of specific stereoisomers. This stereochemical outcome is influenced by the reaction mechanism, the geometry of the reactants, and the steric hindrance around the reaction center. Controlling the stereochemistry is crucial for the synthesis of chiral compounds.
Progress in Nucleophilic Substitution Catalysts
Researchers continue to explore and develop new catalysts to enhance the efficiency and selectivity of nucleophilic substitution reactions. Catalysts can accelerate the reaction rate, improve the regioselectivity and stereochemistry, and enable the use of milder reaction conditions. These advancements open up new possibilities in synthetic chemistry.
Nucleophilic Substitution in Drug Discovery
Nucleophilic substitution reactions are widely used in pharmaceutical research and drug discovery. They are employed in the synthesis of novel drug candidates, modification of lead compounds, and the preparation of intermediates for further chemical transformations. Efficient and reliable nucleophilic substitution methods are crucial for the development of new drugs.
These 15 captivating facts about nucleophilic substitution shed light on the importance and versatility of this fundamental reaction in organic chemistry. From its role in organic synthesis to its involvement in biological systems, nucleophilic substitution plays a crucial part in understanding and manipulating chemical reactions. Exploring the mechanisms, factors affecting the reaction rate, and applications of nucleophilic substitution opens up endless possibilities for advancements in the field of chemistry.
Conclusion
In conclusion, nucleophilic substitution is a fascinating concept in the world of chemistry. It plays a crucial role in organic synthesis, allowing chemists to create new molecules with specific properties. Understanding the mechanisms and factors that influence nucleophilic substitution reactions opens up a wide range of possibilities for researchers and chemists.From the various types of nucleophiles to the different reaction pathways, there is much to explore and discover within this field. By studying nucleophilic substitution, we gain valuable insights into the behavior of organic compounds and how they can be manipulated.Whether you’re a chemistry enthusiast or a student studying the subject, delving deeper into the world of nucleophilic substitution can be an exciting endeavor. The more we understand about this fundamental process, the more we can apply it to various industries, such as pharmaceuticals, materials science, and environmental research.
FAQs
Q: What is nucleophilic substitution?
A: Nucleophilic substitution is a chemical reaction where an incoming nucleophile replaces a leaving group in a molecule. It is a fundamental concept in organic chemistry and plays a crucial role in various chemical processes.
Q: What are nucleophiles?
A: Nucleophiles are electron-rich species that have the ability to donate a pair of electrons to form a new chemical bond. They are attracted to positively charged or electron-deficient atoms and are essential in nucleophilic substitution reactions.
Q: What are the types of nucleophilic substitution reactions?
A: The two main types of nucleophilic substitution reactions are SN1 (unimolecular) and SN2 (bimolecular). In SN1 reactions, the reaction proceeds through a two-step process, while in SN2 reactions, the nucleophile displaces the leaving group in a single, concerted step.
Q: What factors influence nucleophilic substitution reactions?
A: The rate of nucleophilic substitution reactions can be influenced by various factors, including the nature of the nucleophile, the strength of the leaving group, the solvent, and the steric hindrance around the reaction site.
Q: How is nucleophilic substitution used in practical applications?
A: Nucleophilic substitution reactions have numerous practical applications. They are used in the synthesis of pharmaceuticals, the production of polymers, the development of agrochemicals, and many other areas of research and industry.
Q: Can you give an example of a nucleophilic substitution reaction?
A: One example of a nucleophilic substitution reaction is the reaction between an alkyl halide and a hydroxide ion to form an alcohol. The hydroxide ion acts as the nucleophile, displacing the halide ion in the process.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
