Chemistry is a fascinating subject that delves into the intricacies of matter and its transformations. One such fundamental concept in chemistry is substitution reactions. Substitution reactions occur when one atom or group of atoms in a molecule is replaced by another atom or group of atoms. These reactions play a crucial role in various fields, from organic chemistry to industrial applications.
In this article, we will explore 16 astounding facts about substitution reactions. We will delve into the mechanisms involved, the different types of substitutions, and their significance in different areas of chemistry. So, buckle up and get ready to embark on a journey into the world of substitution reactions!
Key Takeaways:
- Substitution reactions are essential in creating new drugs, modifying polymers, and improving food products. They also play a crucial role in environmental protection and the petrochemical industry.
- From DNA mutations to the production of plastics, substitution reactions are everywhere in chemistry. They help create new materials, remove pollutants, and even make our food healthier!
The Origin of Substitution
Substitution, a fundamental concept in chemistry, traces its roots back to the early 19th century. The term was coined by French chemist Claude Louis Berthollet, who proposed the idea that chemical reactions involve the replacement of one element or group of elements with another.
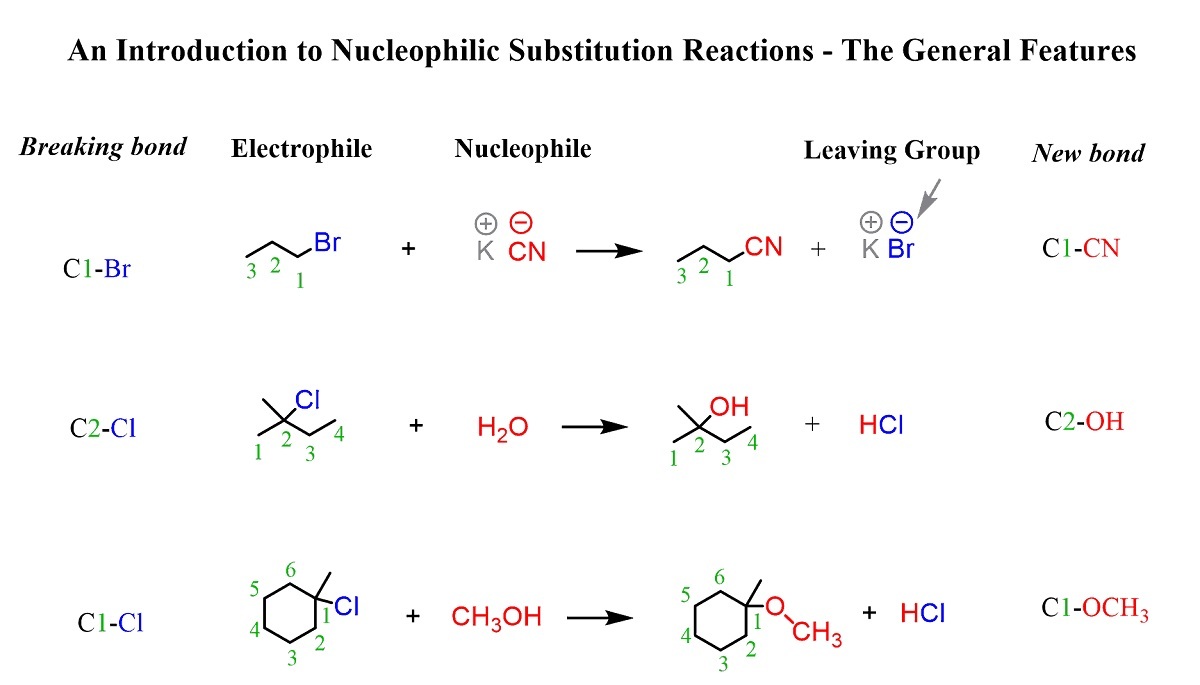
Substitution in Organic Chemistry
Organic chemistry heavily relies on substitution reactions. In these reactions, an atom or a group of atoms is replaced by another atom or group of atoms. It plays a crucial role in the synthesis of various organic compounds and the understanding of their chemical properties.
Types of Substitution Reactions
There are several types of substitution reactions, including nucleophilic substitution, electrophilic substitution, radical substitution, and free radical substitution. Each type has its own unique mechanism and set of reaction conditions.
Application in Pharmaceutical Industry
The field of pharmaceuticals extensively utilizes substitution reactions. Pharmaceutical scientists employ substitution reactions to introduce functional groups or modify existing molecular structures, aiming to create new drugs with enhanced efficacy and reduced side effects.
Role in Polymer Science
In polymer science, substitution reactions are used to modify polymer chains and create new materials with tailored properties. Substitution plays a vital role in the synthesis of polymers such as polyethylene, polypropylene, and polystyrene.
Substitution in Biochemistry
In biochemistry, substitution reactions are involved in processes like DNA replication and repair. Substitution of nucleotides in DNA plays a critical role in genetic mutations and variations.
Halogenation as a Substitution Reaction
Halogenation is a common type of substitution reaction where a halogen atom replaces another atom in a compound. This reaction is widely used in the synthesis of various organic compounds, including pharmaceuticals, pesticides, and plastics.
Environmental Implications of Substitution
Substitution reactions can have significant environmental implications. For instance, the introduction of less harmful substances through substitution helps in reducing pollution and minimizing the impact of hazardous chemicals on ecosystems.
Substitution in Inorganic Chemistry
Substitution reactions are not limited to organic compounds but also occur in inorganic chemistry. In these reactions, an atom or ion is replaced by another atom or ion in a compound. This process plays a vital role in various chemical reactions and transformations.
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution is a type of substitution reaction that occurs specifically on aromatic compounds. It involves the replacement of a hydrogen atom by an electrophile, resulting in the formation of a new aromatic compound.
Substitution in Food Science
Substitution reactions are utilized in food science to enhance the nutritional content and improve the sensory characteristics of food products. For example, the substitution of ingredients can lead to the development of healthier alternatives.
Substitution Reactions in Medicinal Chemistry
Medicinal chemists rely on substitution reactions to introduce specific functional groups into drug molecules, enhancing their potency and selectivity. Substitution plays a crucial role in the development of new drugs and pharmaceutical formulations.
Nucleophilic Substitution in Alcohols
Nucleophilic substitution reactions in alcohols involve the replacement of the hydroxyl group (-OH) by a nucleophile. This type of reaction is widely used in organic synthesis and plays a key role in the production of various organic compounds.
Importance in Petrochemical Industry
The petrochemical industry heavily relies on substitution reactions for the production of various chemicals and polymers from petroleum-based feedstocks. These reactions enable the transformation of crude oil into valuable products like plastics, fuels, and solvents.
Substitution as a Tool in Analytical Chemistry
Substitution reactions find applications in analytical chemistry. They can be employed to selectively convert a specific component of a sample into a different compound for detection or quantification, enabling accurate analysis and identification of substances.
Substitution in Environmental Remediation
Substitution reactions are employed in environmental remediation processes to remove or transform harmful pollutants. By substituting contaminants with less toxic substances, these reactions help in restoring and improving the quality of soil and water.
Conclusion
In conclusion, substitution is an incredibly fascinating aspect of chemistry. With its wide range of applications and profound impact on chemical reactions, understanding the concept of substitution opens up a whole new world of possibilities. From the discovery of new compounds to the development of life-saving drugs, substitution plays a crucial role in advancing the field of chemistry.
By replacing one atom or group of atoms with another, chemists are able to alter the properties and behavior of compounds, leading to the creation of new materials and substances. Substitution reactions occur in various branches of chemistry, including organic, inorganic, and biochemistry.
Whether you’re a student, a researcher, or simply have an interest in the subject, delving deeper into the world of substitution will undoubtedly enhance your understanding and appreciation of chemistry as a whole.
FAQs
1. What is substitution in chemistry?
Substitution in chemistry refers to the replacement of one atom or group of atoms in a molecule with another. It is a fundamental concept that plays a vital role in the synthesis of new compounds and the modification of existing molecules.
2. What are some examples of substitution reactions?
Some common examples of substitution reactions include the halogenation of alkanes, where a hydrogen atom in the alkane is replaced by a halogen atom, and the esterification process, where the hydroxyl group in an alcohol is substituted by an alkyl or acyl group.
3. How does substitution affect the properties of compounds?
Substitution can significantly alter the properties of compounds. For instance, substituting a functional group with a more electronegative group can increase the acidity of a compound. Additionally, substitutions can affect the reactivity, stability, and solubility of compounds.
4. What are the applications of substitution reactions?
Substitution reactions have numerous applications in various fields. They are widely used in drug discovery and development, as well as in the synthesis of complex organic molecules. Substitution reactions also play a crucial role in industrial processes, such as the production of plastics, dyes, and pharmaceuticals.
5. How does substitution contribute to the advancement of chemistry?
Substitution reactions pave the way for the discovery of new compounds and the development of innovative materials. By substituting atoms or groups of atoms in molecules, chemists are able to explore new chemical pathways, study the reactivity of different compounds, and ultimately contribute to the advancement of knowledge in the field of chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


