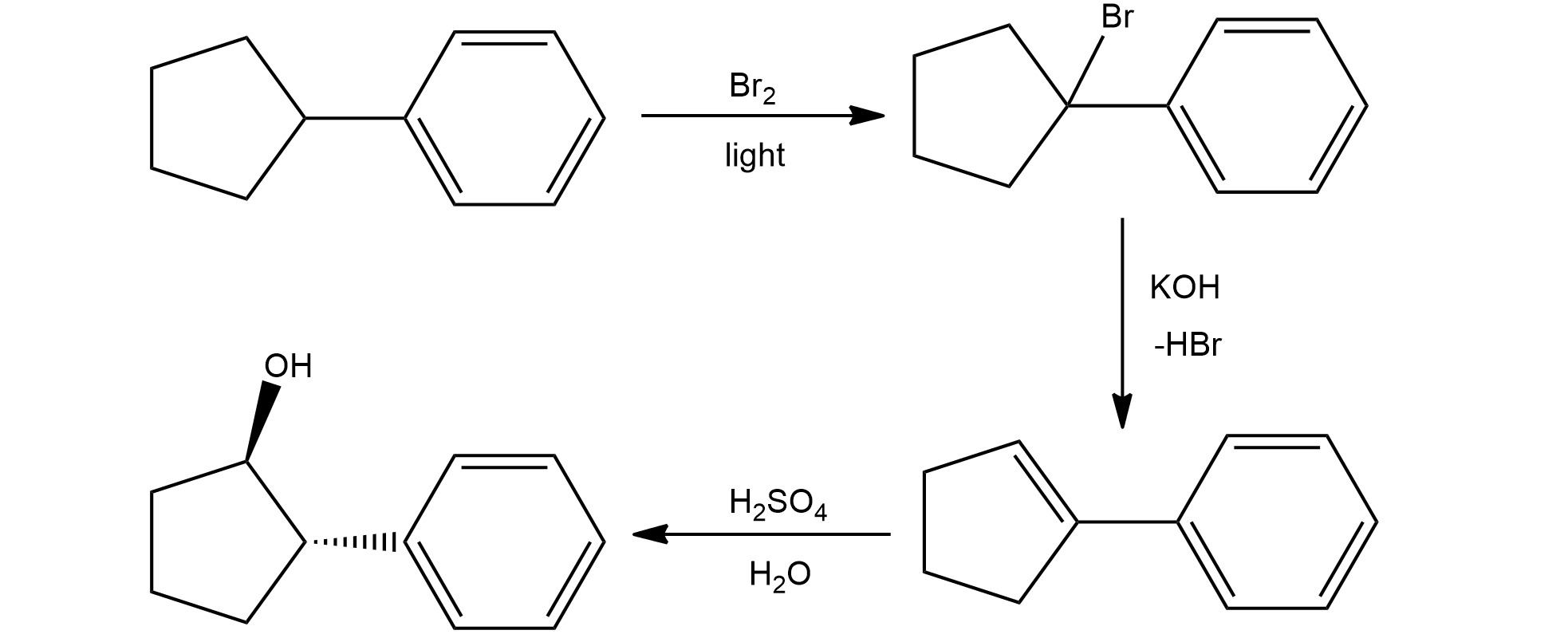
Retrosynthetic analysis, also known as retro- synthesis, is a powerful problem-solving tool in the field of organic chemistry. It involves breaking down complex molecules into simpler building blocks, allowing chemists to design efficient synthetic routes for the production of target compounds. While retrosynthetic analysis may seem daunting to beginners, it is an essential skill that can greatly enhance a chemist’s ability to plan and execute organic syntheses.
In this article, we will delve into the fascinating world of retrosynthetic analysis and uncover 17 surprising facts that will not only deepen your understanding of this technique but also pique your curiosity about its applications in drug discovery, natural product synthesis, and materials science. Whether you are a chemistry enthusiast, a student, or a seasoned chemist, prepare to be amazed by the versatility and ingenuity of retrosynthetic analysis!
Key Takeaways:
- Retrosynthetic analysis, developed by Elias James Corey, helps chemists plan efficient synthetic routes by breaking down complex molecules and considering different strategies, fostering creativity and problem-solving skills.
- Computer programs aid in retrosynthetic analysis, which is crucial in drug discovery and natural product synthesis. It helps chemists optimize synthetic routes, visualize possibilities, and consider regioselectivity and stereoselectivity.
Retrosynthetic analysis was developed by Nobel laureate Elias James Corey.
Elias James Corey, an American chemist, introduced the concept of retrosynthetic analysis in the late 1960s. His groundbreaking work revolutionized the field of organic synthesis.
It involves breaking down complex molecules into simpler fragments.
Retrosynthetic analysis involves breaking down a target molecule into smaller fragments called synthons. These synthons represent the building blocks that can be used to reconstruct the target molecule.
The process relies on functional group interconversions.
Functional group interconversions are key steps in retrosynthetic analysis. By transforming one functional group into another, chemists can manipulate the structure of the target molecule and identify potential synthetic pathways.
Retrosynthetic analysis helps identify potential synthetic routes.
By systematically analyzing a target molecule, chemists can determine multiple synthetic routes to its synthesis. This allows for flexibility and optimization in the laboratory.
Computer programs can aid in retrosynthetic analysis.
Modern computational tools have been developed to assist chemists in performing retrosynthetic analysis. These programs utilize databases and algorithms to suggest possible synthetic pathways.
Different retrosynthetic strategies can be employed.
Chemists can employ various strategies during retrosynthetic analysis, such as disconnection-based retrosynthesis, bioisosterism, and functional group interchange. Each approach offers different insights and solutions.
Retrosynthetic analysis is used in drug discovery.
Retrosynthetic analysis is an essential tool in the development of new pharmaceuticals. It helps chemists design efficient synthetic routes to target molecules, leading to the creation of novel drugs.
The process considers regioselectivity and stereoselectivity.
During retrosynthetic analysis, chemists must consider the regioselective and stereoselective aspects of the reaction. This ensures that the desired stereochemistry and regiochemistry are achieved in the synthesis.
Forward synthesis complements retrosynthetic analysis.
Forward synthesis, the conventional approach of synthesizing molecules from simple starting materials, works hand in hand with retrosynthetic analysis. The two methods complement each other to achieve efficient synthesis.
Retrosynthetic analysis aids in the optimization of synthetic routes.
By analyzing the retrosynthetic tree, chemists can identify potential bottlenecks and modifications to optimize the synthetic route. This leads to improved yields and reduced production costs.
It has found applications in natural product synthesis.
Retrosynthetic analysis has proven indispensable in the synthesis of complex natural products. Chemists can break down intricate molecules found in nature into simpler building blocks for synthesis.
The concept can be applied to retrosynthetic planning of polymers.
Retrosynthetic analysis can also be applied to the synthesis of polymers. Chemists can analyze the repeat units and functional groups in a polymer chain to plan efficient synthetic routes.
It facilitates retrosynthesis-based retrospective analysis.
Retrosynthetic analysis can be used to trace the steps taken to synthesize a molecule by working backwards. This retrosynthesis-based retrospective analysis helps in identifying and rectifying any synthetic errors.
Retrosynthetic analysis considers protecting groups.
Protecting groups are temporary modifications made to functional groups to prevent unwanted reactions during synthesis. Retrosynthetic analysis takes into account the strategic use of protecting groups.
It is a crucial tool in total synthesis.
Retrosynthetic analysis plays a vital role in total synthesis, which involves the complete construction of complex molecules from simple starting materials. It guides chemists in designing efficient and practical synthetic routes.
The retrosynthetic tree helps visualize different possibilities.
The retrosynthetic tree is a graphical representation of the potential synthetic pathways derived from retrosynthetic analysis. It helps chemists explore different possibilities and evaluate various synthetic options.
Retrosynthetic analysis fosters creativity and problem-solving skills.
Retrosynthetic analysis challenges chemists to think critically, creatively, and strategically to devise efficient synthetic routes. It enhances problem-solving skills and encourages innovation in organic synthesis.
These 17 surprising facts about retrosynthetic analysis demonstrate its immense importance and impact in the field of organic chemistry. From drug discovery to natural product synthesis, retrosynthetic analysis continues to revolutionize the way chemists plan and execute complex synthesis processes.
Conclusion
In conclusion, retrosynthetic analysis is a powerful tool in organic chemistry that allows chemists to work backwards from a target molecule to identify possible synthetic routes. It involves breaking down complex molecules into simpler fragments, which can then be reassembled using known chemical reactions. This approach not only saves time and resources in the laboratory but also enables chemists to design more efficient and sustainable synthetic strategies.Through retrosynthetic analysis, chemists can uncover unexpected connections between different molecules and explore innovative methods for synthesizing complex structures. By applying this approach, researchers have made remarkable discoveries and advancements in drug development, materials science, and other areas of chemical research.As we continue to deepen our understanding of retrosynthetic analysis, we can expect even more exciting possibilities in organic synthesis. This field provides endless opportunities for creativity and innovation, allowing chemists to push the boundaries of what is possible in the world of chemistry.
FAQs
Q: What is retrosynthetic analysis?
A: Retrosynthetic analysis is a technique in organic chemistry that involves breaking down complex molecules into simpler fragments to identify possible synthetic routes.
Q: Why is retrosynthetic analysis important?
A: Retrosynthetic analysis allows chemists to plan and design synthetic routes for complex molecules, saving time and resources in the laboratory and enabling the development of efficient and sustainable synthetic strategies.
Q: How does retrosynthetic analysis work?
A: Retrosynthetic analysis works by starting with a target molecule and breaking it down into simpler fragments using known chemical reactions. These fragments can then be reassembled to synthesize the target molecule.
Q: What are the applications of retrosynthetic analysis?
A: Retrosynthetic analysis finds applications in drug development, materials science, and other areas of chemical research where the synthesis of complex molecules is required.
Q: Can retrosynthetic analysis lead to unexpected discoveries?
A: Yes, retrosynthetic analysis can lead to unexpected discoveries by revealing connections between different molecules and suggesting novel synthetic approaches.
Q: Does retrosynthetic analysis have limitations?
A: Retrosynthetic analysis relies on the availability of known chemical reactions and can be limited by the complexity of the molecule being analyzed.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
