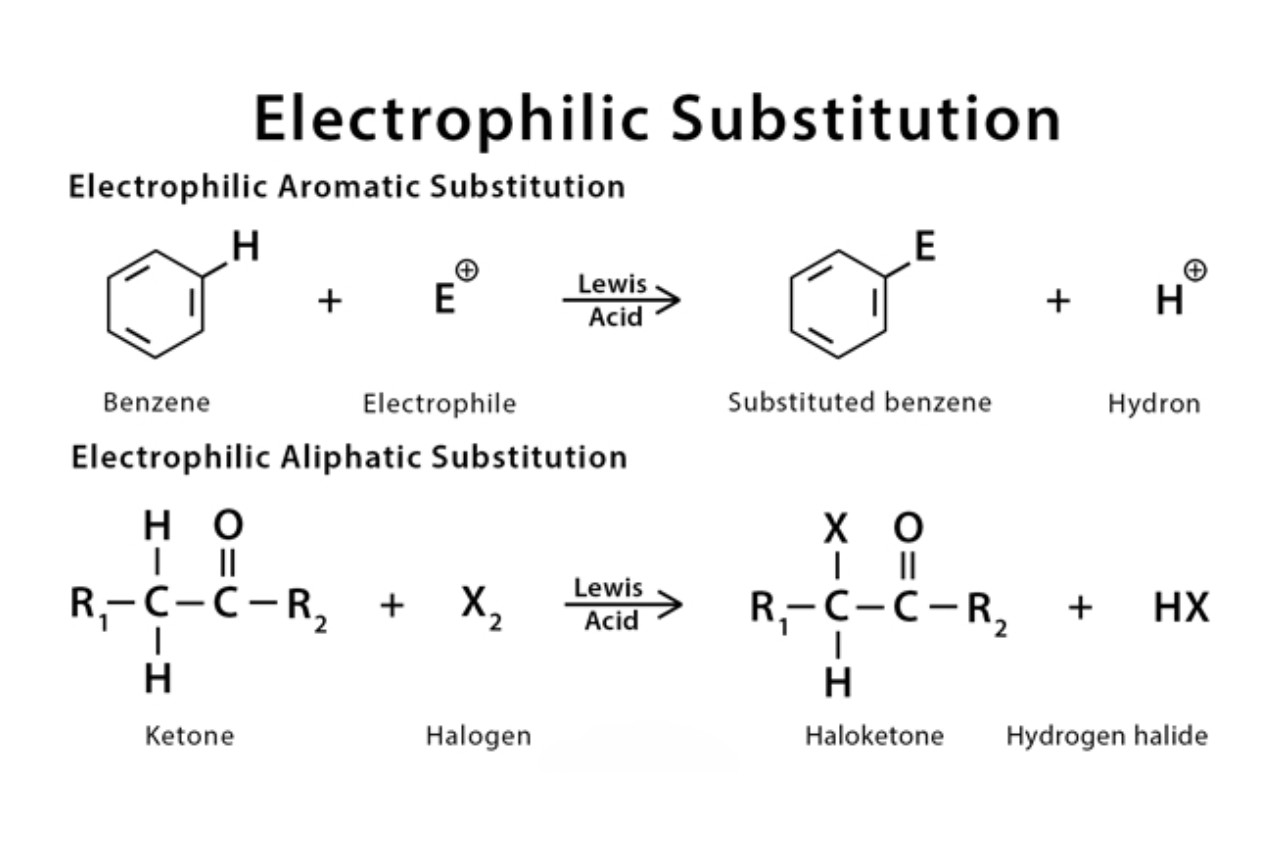
Electrophilic substitution is a fundamental concept in chemistry that plays a crucial role in understanding various organic reactions. It occurs when an electrophile, a species that seeks electrons, replaces a hydrogen atom in an organic compound. This process leads to the formation of a new product with different properties and functionalities. Electrophilic substitution reactions are not only important for synthesizing complex organic molecules but also have practical applications in pharmaceuticals, materials science, and other industries.
In this article, we will delve into the fascinating world of electrophilic substitution and explore some extraordinary facts about this phenomenon. From the mechanisms behind these reactions to their diverse applications, we will uncover intriguing insights that highlight the significance of electrophilic substitution in the field of chemistry. So, let’s embark on this journey and discover the remarkable aspects of electrophilic substitution reactions!
Key Takeaways:
- Electrophilic substitution is like a game of musical chairs in organic compounds, where an electron-hungry guest replaces an existing atom. It’s crucial for making new molecules and understanding their behavior.
- Just like adding toppings to a pizza, electrophilic substitution reactions can lead to different products and flavors. They’re like a secret recipe for creating new medicines and materials.
Electrophilic substitution is a fundamental concept in organic chemistry.
Electrophilic substitution refers to a chemical reaction in which an electrophile replaces an existing group or atom in an organic compound. It plays a crucial role in the synthesis and transformation of organic molecules.
Electrophilic substitution reactions involve the presence of electron-deficient species.
In electrophilic substitution, the electrophile is attracted to the electron-rich region of the organic molecule, leading to the substitution of a functional group. This process relies on the electrophile’s ability to accept an electron pair.
Aromatic compounds are particularly susceptible to electrophilic substitution.
Aromatic compounds, such as benzene, are highly reactive towards electrophiles due to their delocalized electron systems. This property makes them ideal candidates for electrophilic substitution reactions.
Electrophilic substitution can occur through multiple mechanisms.
Common mechanisms for electrophilic substitution include the addition-elimination mechanism and the elimination-addition mechanism. The choice of mechanism depends on the specific reaction conditions and the nature of the electrophile and substrate.
Nitration is a well-known example of electrophilic substitution.
In nitration, a nitro group (-NO2) is introduced into an organic compound by replacing an existing hydrogen atom. This reaction is commonly used in the synthesis of explosives, dyes, and pharmaceuticals.
Halogenation is another important electrophilic substitution reaction.
Halogenation involves the introduction of a halogen atom (-Cl, -Br, -I) into an organic molecule. This reaction is widely used in organic synthesis to prepare compounds with specific halogen substitutions.
Electrophilic aromatic substitution can result in isomer formation.
In some cases, electrophilic aromatic substitution can lead to the formation of different isomers due to the position of the substituents. This isomerism adds complexity and diversity to the field of organic chemistry.
Electrophilic substitution reactions are influenced by the electron-donating and electron-withdrawing nature of substituents.
The presence of electron-donating or electron-withdrawing groups on the aromatic ring can greatly influence the reactivity of electrophilic substitution reactions. These substituents can either enhance or hinder the substitution process.
Friedel-Crafts reactions are a classic example of electrophilic substitution.
Friedel-Crafts reactions involve the substitution of an aromatic ring with an alkyl or acyl group. These reactions have broad applications in the synthesis of various organic compounds, including flavors, fragrances, and pharmaceuticals.
Electrophilic substitutions can occur on heteroaromatic compounds.
Heteroaromatic compounds, which contain atoms other than carbon within the aromatic ring, can undergo electrophilic substitution reactions. Examples include pyridine, pyrrole, and furan.
Electrophilic substitution reactions can be regioselective.
The regioselectivity of electrophilic substitution refers to the preference for a specific site on the aromatic ring for the electrophilic attack. This selectivity is often influenced by the electron density and the steric hindrance around the ring.
Electrophilic substitution reactions can lead to the formation of multiple products.
Depending on the conditions and nature of the reactants, electrophilic substitution can result in the formation of multiple products with different substitution patterns. Careful control of reaction conditions is necessary to achieve the desired product.
Electrophilic substitution reactions are widely used in the synthesis of pharmaceuticals.
Electrophilic substitution plays a crucial role in the synthesis of many pharmaceutical compounds. It allows chemists to introduce specific functional groups into organic molecules, enabling the development of new drugs and therapies.
Electrophilic substitution reactions can be catalyzed by Lewis acids.
Lewis acids, such as aluminum chloride or iron(III) chloride, can act as catalysts in electrophilic substitution reactions. They help facilitate the formation of the electrophile and enhance the reaction rate.
Electrophilic substitution reactions are reversible under certain conditions.
Under specific conditions, electrophilic substitution reactions can be reversed, leading to the regeneration of the starting material. This reversibility adds another dimension to the complexity of these reactions.
Electrophilic substitution reactions are extensively studied in organic chemistry research.
Due to their importance and wide applicability, electrophilic substitution reactions are a subject of intense investigation in the field of organic chemistry. Researchers are continuously exploring new reagents and techniques to improve the efficiency and selectivity of these reactions.
Electrophilic substitution reactions are essential in understanding and predicting the behavior of organic compounds.
By studying electrophilic substitution reactions, chemists can gain valuable insights into the reactivity and behavior of organic compounds. This knowledge is crucial for designing efficient synthetic routes and developing new materials and technologies.
Conclusion
In conclusion, electrophilic substitution is a fascinating and important concept in organic chemistry. Through the process of electrophilic substitution, compounds can undergo unique reactions that result in the replacement of one atom or group with another. This mechanism plays a vital role in the synthesis and modification of numerous organic compounds, making it a key area of study for chemists.
By understanding electrophilic substitution and its various reactions, researchers can develop new drugs, design more efficient catalysts, and unlock the potential of organic synthesis. The 17 extraordinary facts about electrophilic substitution discussed in this article shed light on the diverse nature of this mechanism and its applications in various fields. Whether you are a chemistry enthusiast or a professional chemist, exploring the world of electrophilic substitution is sure to be an exciting and rewarding journey.
FAQs
1. What is electrophilic substitution?
Electrophilic substitution is a type of reaction in organic chemistry, where an electrophile attacks an atom or group of atoms in a molecule, leading to the replacement of the existing atom or group. This mechanism is commonly observed in aromatic compounds.
2. How does electrophilic substitution occur?
Electrophilic substitution occurs through the attack of an electrophile, which is an electron-deficient species, on a nucleophilic site in the substrate molecule. The electrophile forms a bond with the nucleophile, resulting in the substitution of the original atom or group in the molecule.
3. What are some examples of electrophilic substitution reactions?
Common examples of electrophilic substitution reactions include nitration, halogenation, sulfonation, and Friedel-Crafts reactions. In these reactions, the electrophile replaces a hydrogen atom in an aromatic compound, resulting in the introduction of a new functional group.
4. How is electrophilic substitution important in organic synthesis?
Electrophilic substitution plays a crucial role in organic synthesis as it allows for the introduction of various functional groups onto aromatic compounds. This enables chemists to tailor the properties of organic molecules, such as their reactivity, solubility, and bioactivity, leading to the development of new drugs, materials, and chemicals.
5. Can electrophilic substitution occur in non-aromatic compounds?
While electrophilic substitution is most commonly observed in aromatic compounds, it can also occur in non-aromatic systems under specific conditions. However, the reactivity and mechanism may differ from those observed in aromatic electrophilic substitution.
6. Are there any limitations or challenges associated with electrophilic substitution?
One limitation of electrophilic substitution is the regioselectivity, where multiple positions on the aromatic ring may be susceptible to substitution. Additionally, some electrophilic substitution reactions may require harsh reaction conditions or the use of toxic reagents, posing challenges for large-scale industrial applications.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
