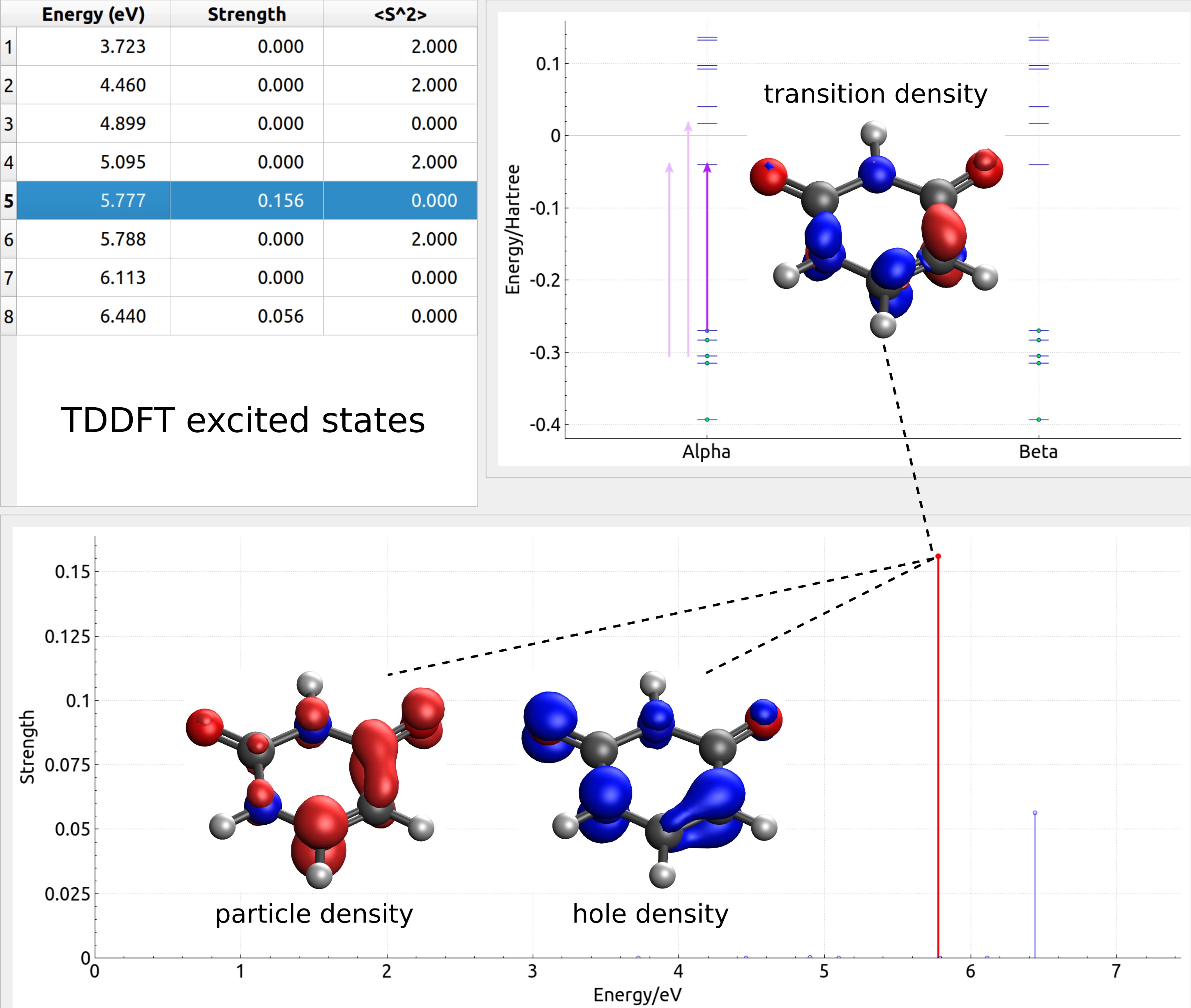
The excited state is a fascinating concept in the field of chemistry that showcases the dynamic nature of atoms and molecules. When an atom or a molecule absorbs energy, it transitions from its ground state, or the lowest energy state, to an excited state. This process leads to a variety of remarkable phenomena that have captivated scientists for centuries.
In this article, we will delve into the world of excited states and uncover 16 astounding facts that will further enhance our understanding of this intriguing topic. From the role of excited states in fluorescence and phosphorescence to their significance in chemical reactions and spectroscopy, prepare to be amazed by the wonders of the excited state.
Key Takeaways:
- Excited states make atoms and molecules act like they’ve had too much coffee, getting all energetic and causing cool things like glowing and chemical reactions. It’s like a chemistry party!
- Excited states are like superheroes in chemistry, helping with things like capturing sunlight for plants, making glow-in-the-dark toys, and even powering lasers. They’re the real MVPs of the chemical world!
What is the Excited State?
The excited state is a term commonly used in chemistry to describe a higher-energy state that an atom, molecule, or subatomic particle can assume when it absorbs energy. In this state, the electron configuration of the atom or molecule is altered, leading to changes in its physical and chemical properties.
Electron Promotion
In the excited state, electrons may be promoted from their ground state to higher energy levels or different orbitals. This transition occurs when atoms or molecules absorb energy, typically in the form of photons, causing the electrons to jump to higher energy levels.
Emission of Light
When electrons return to their lower energy levels from the excited state, they release the excess energy as photons of light. This emission of light is the basis for various phenomena such as fluorescence, phosphorescence, and luminescence.
Role in Spectroscopy
The excited state plays a crucial role in spectroscopic techniques like UV-Vis spectroscopy and fluorescence spectroscopy. These methods utilize the absorption and emission of light to study the energy levels and transitions of molecules.
Excited State Lifetimes
The excited state of a species can have different lifetimes, ranging from nanoseconds to microseconds or even longer. The duration of the excited state is determined by several factors, including the nature of the species and the surrounding environment.
Energy Absorption in Excited State
When an atom or molecule absorbs energy and reaches an excited state, it becomes more reactive and can participate in chemical reactions that are typically not possible in the ground state. These reactions play a significant role in photochemistry and photobiology.
Role in Photosynthesis
In photosynthesis, the excited state of chlorophyll molecules plays a pivotal role. It allows them to capture sunlight and convert it into chemical energy, which is then used to drive the process of photosynthesis in plants and other photosynthetic organisms.
Excited State in Luminescent Materials
Many luminescent materials, such as glow-in-the-dark toys and fluorescent dyes, contain compounds that can be excited to higher energy states. These materials emit light when the excited electrons return to their ground state, creating the vibrant glow we see.
Quantum Mechanics and Excited State
The behavior of atoms and molecules in the excited state is governed by the principles of quantum mechanics. Quantum mechanical calculations and theories help scientists understand and predict the energy levels, transitions, and properties of excited states.
Biological Significance
The excited state is of significant biological importance as it is involved in processes such as vision, DNA damage and repair, and the functioning of certain proteins. Understanding the behavior of excited states in biological systems has immense implications for medical and biochemical research.
Excited States in Flame Spectroscopy
Flame spectroscopy utilizes the excited states of atoms and ions in flames to analyze their elemental composition. By observing the characteristic emission spectra, scientists can identify and quantify the presence of different elements in a sample.
Excited States in Lasers
Laser technology heavily relies on the principles of excited states. Excited atoms or molecules in a laser system undergo stimulated emission, allowing for the amplification of light and the production of a coherent and intense beam of light.
Excited State Decay Pathways
When in the excited state, atoms and molecules can decay back to their ground state through various pathways. These include non-radiative processes, where energy is released as heat, or radiative processes, where photons are emitted.
Multiple Excited States
Some atoms and molecules can have multiple excited states with different energy levels. The presence of multiple excited states can give rise to complex energy level diagrams and transitions, leading to unique spectroscopic signatures.
Excited State Interactions
Excited states can interact with each other, leading to phenomena such as energy transfer, quenching, and exciton dynamics. These interactions play a crucial role in fields like solar cells, where the efficient transfer of energy between excited states is desirable.
Excited States in Chemical Reactions
The excited states of reactants and intermediates are involved in numerous chemical reactions, including photochemical reactions. Excited-state reactions enable the formation of new bonds, the breaking of existing bonds, and unique reaction pathways that are not accessible in the ground state.
In conclusion, the excited state is a captivating concept in chemistry that governs the behavior and properties of atoms, molecules, and subatomic particles. With its role in spectroscopy, photosynthesis, luminescence, and various chemical reactions, understanding the excited state is vital for advancing our knowledge and applications in fields ranging from materials science to biochemistry.
Conclusion
In conclusion, the concept of excited state in chemistry is truly fascinating. It is a state in which atoms or molecules have absorbed energy and moved to higher energy levels. This phenomenon plays a crucial role in understanding various chemical reactions, as well as in the fields of spectroscopy and photochemistry.By delving into the depths of excited state properties and behavior, researchers have unlocked a plethora of astounding facts. From the emission of colorful light in fireworks to the functioning of solar cells, excited states have far-reaching implications in our everyday lives.Not only does studying excited states provide us with a deeper understanding of fundamental chemical processes, but it also paves the way for technological advancements in areas such as materials science, drug development, and energy conversion.In summary, the study of excited state continues to captivate scientists and fuel groundbreaking research. Exploring this dynamic aspect of chemistry has the potential to unlock unimaginable possibilities and shape the future of scientific discovery.
FAQs
Q: What is an excited state in chemistry?
A: An excited state in chemistry refers to a state in which atoms or molecules have absorbed energy and moved to higher energy levels. This occurs when electrons transition from their ground state to an excited state through the absorption of energy.
Q: How do atoms or molecules become excited?
A: Atoms or molecules become excited by absorbing energy, typically in the form of photons. This energy excites the electrons, causing them to move to higher energy levels.
Q: What happens when an excited state returns to the ground state?
A: When an excited state returns to the ground state, the excess energy is released. This energy can be emitted as light, resulting in phenomena such as fluorescence or phosphorescence, or it can be dissipated as heat.
Q: What are some practical applications of excited states?
A: Excited states have numerous practical applications. They are crucial in fields such as spectroscopy, which involves the study of the interaction between matter and electromagnetic radiation. Excited states also play a major role in technologies like lasers, solar cells, and photodynamic therapy used in cancer treatment.
Q: Can excited states be observed directly?
A: While excited states themselves cannot be observed directly, their effects can be observed and measured through various spectroscopy techniques. These techniques allow scientists to study the behavior and properties of excited states indirectly by analyzing the emitted or absorbed electromagnetic radiation.
Excited states play a crucial role in chemistry, but there's more to explore! Delve into plasma physics and its astounding facts. Unravel the mysteries of this fascinating field, where charged particles and electromagnetic forces interplay. Plasma physics holds the key to understanding the universe, from stars to fusion energy. Join us on this thrilling journey of discovery and expand your knowledge beyond the realms of excited states. Get ready to be amazed by the wonders of plasma physics and the groundbreaking insights it offers. Don't miss out on this opportunity to broaden your scientific horizons!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
