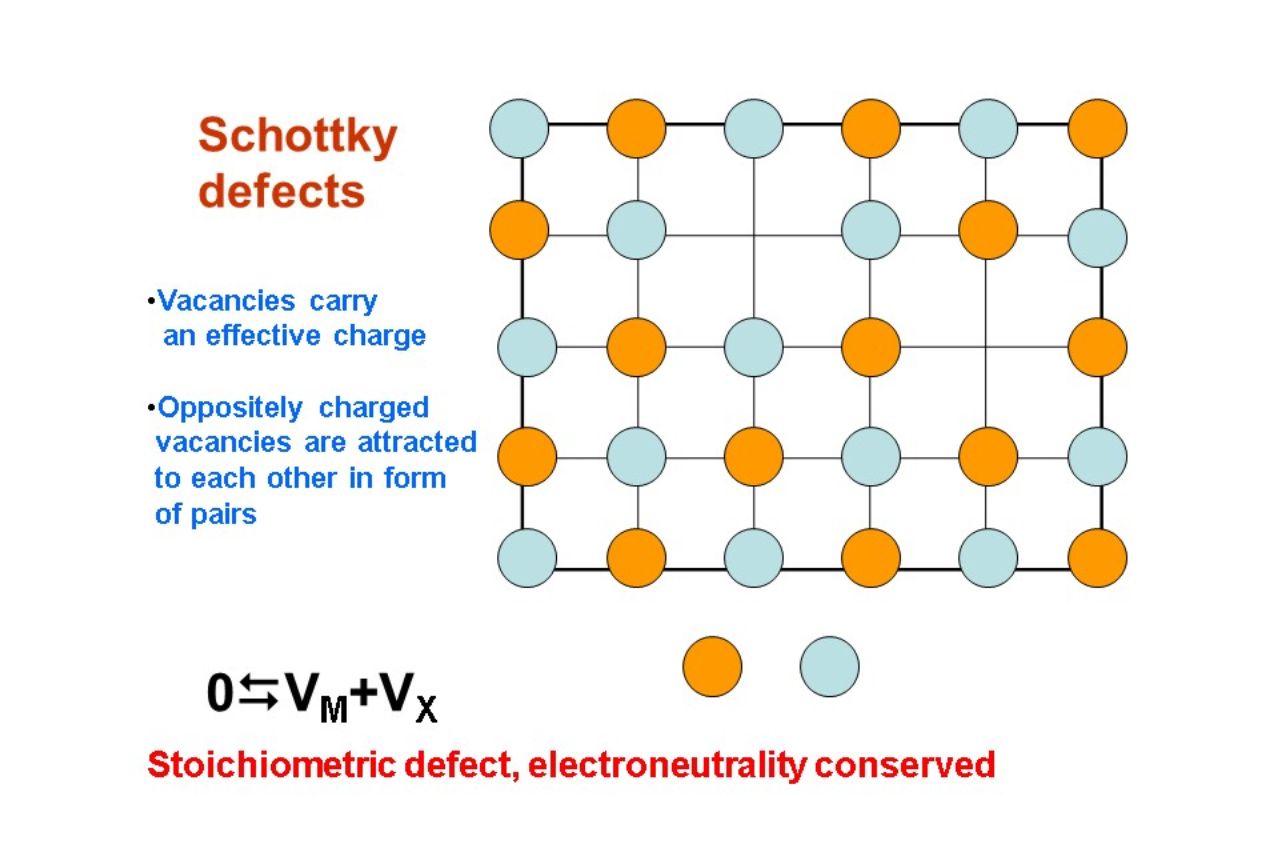
Schottky defect is a fascinating concept in the field of solid-state chemistry that has intrigued researchers and scientists for decades. It is a type of point defect that occurs in crystalline materials, causing a deviation from the ideal atomic arrangement. This defect is named after Walter H. Schottky, a German physicist who made significant contributions to solid-state physics.
In this article, we will explore 19 fascinating facts about Schottky defect, shedding light on its properties, formation, and impact on the physical and chemical properties of materials. From its discovery to its applications in various fields, Schottky defect plays a crucial role in understanding the behavior of crystalline substances.
So, grab your lab coat and join us on this intriguing journey as we unlock the mysteries of Schottky defect and dive into the world of solid-state chemistry.
Key Takeaways:
- Schottky defects are vacancies in crystal structures that impact material properties like conductivity and stability, crucial in designing semiconductor devices and energy storage. They can also influence crystal cleavage and catalytic processes.
- Scientists study Schottky defects to improve material properties and develop new materials. These defects affect ionic migration, crystal stability, and energy conversion in solid oxide fuel cells, making them essential in material science and energy technology.
The Discovery of the Schottky Defect
The Schottky defect, also known as the Schottky imperfection, was first discovered by Walter H. Schottky in the early 1900s. He observed the presence of vacancies in lattice structures, specifically in ionic compounds.
Defects in Ionic Crystals
The Schottky defect is a common type of defect found in ionic crystals. It occurs when lattice positions normally occupied by ions become vacant, leading to a decrease in the crystal’s density.
Formation of Schottky Defect
Schottky defects can be formed during the crystal growth process due to the difference in size between cations and anions. The ions may not occupy their predetermined positions, resulting in vacant sites.
Importance in Solid State Chemistry
The study of Schottky defects is crucial in the field of solid-state chemistry. It provides insights into the behavior and properties of ionic materials, including conductivity and stability.
Impact on Electrical Conductivity
The presence of Schottky defects can significantly affect the electrical conductivity of ionic materials. The creation of vacancies disrupts the regular flow of ions, leading to changes in conductivity.
Influence on Material Properties
Schottky defects can alter various material properties, such as optical and magnetic properties. These defects can create localized states within the material, influencing its behavior.
Role in Semiconductor Devices
The control and manipulation of Schottky defects are essential in the design and functioning of semiconductor devices. Engineers utilize these defects to modify the electrical properties of the devices.
Temperature Dependence of Schottky Defects
Schottky defects exhibit temperature-dependent behavior. As temperature increases, the concentration of defects tends to increase due to enhanced atomic mobility.
Relationship to Point Defects
Schottky defects are classified as point defects, which are atomic or ionic irregularities in crystal structures. Point defects also include vacancies, interstitials, and Frenkel defects.
Influence on Ionic Migration
The presence of Schottky defects enhances the ionic migration capabilities of materials. The ionic conductivity of a crystal is dependent on the concentration and mobility of these defects.
Impact on Crystal Stability
Schottky defects can affect the stability of an ionic crystal. The creation of vacancies leads to a decrease in the crystal’s energy, making it more prone to structural changes.
Role in Energy Storage Devices
Schottky defects play a vital role in energy storage devices such as batteries and fuel cells. These defects affect the transport of ions and can influence device performance.
Exploration in Material Science
Scientists and researchers continue to explore Schottky defects in material science. By understanding and controlling these defects, they can develop new materials with improved properties.
Influence on Crystal Cleavage
Schottky defects can influence the ease of crystal cleavage. The presence of vacancies weakens the crystal structure, making it more susceptible to fracture.
Role in Defect Engineering
Defect engineering involves intentionally introducing defects into materials for specific purposes. Schottky defects are one of the defects that can be engineered to enhance material properties.
Relationship with Stoichiometry
Schottky defects are closely related to the stoichiometry of a material. The formation of vacancies disrupts the perfect ratio of ions, leading to deviations from the ideal stoichiometric composition.
Interaction with Other Defects
Schottky defects can interact with other types of defects present in a crystal lattice, such as interstitials or Frenkel defects. These interactions can influence material behavior and properties.
Role in Solid Oxide Fuel Cells
Schottky defects are critical in the operation of solid oxide fuel cells. These defects facilitate the movement of ions, enabling efficient energy conversion processes.
Application in Catalysis
Schottky defects play a significant role in catalytic processes. The presence of these defects enhances the exchange of ions, promoting surface reactions and improving catalytic performance.
Conclusion
In conclusion, Schottky defect is an intriguing phenomenon in solid-state chemistry that plays a crucial role in determining the properties and behavior of materials. Understanding the fascinating facts about Schottky defect is essential for researchers and students alike to delve deeper into the fascinating world of crystal defects.Schottky defects occur when ions or vacancies are missing from their regular lattice positions in a crystalline structure. These defects can significantly affect the electrical and optical properties of materials, making them of great interest in various fields such as electronics, catalysis, and materials science.The 19 fascinating facts about Schottky defect discussed in this article shed light on the complexity and importance of this phenomenon. From its discovery by Walter H. Schottky in the early 20th century to its applications in modern technologies, Schottky defect continues to intrigue scientists and drive advancements in materials research.By further exploring Schottky defect and its implications, researchers can uncover new insights into the behavior of materials, leading to the development of innovative technologies and improved material design.
FAQs
Q: What is a Schottky defect?
A: A Schottky defect is a type of crystal defect that occurs when oppositely charged ions within a crystal structure are missing from their regular lattice positions.
Q: What causes Schottky defects?
A: Schottky defects are caused by vacancies, which are empty lattice sites within the crystal structure. These vacancies can result from a variety of factors, such as thermal energy or impurities in the crystal.
Q: What are the effects of Schottky defects on material properties?
A: Schottky defects can significantly impact the electrical and ionic conductivity, as well as the optical properties of materials. They can alter the energy band structure and introduce new energy states, affecting the overall behavior of the material.
Q: How are Schottky defects detected?
A: Schottky defects can be detected and studied using various experimental techniques, such as X-ray diffraction, electron microscopy, and spectroscopy. These methods allow scientists to visualize and analyze the crystal structure, identifying any deviations caused by the presence of Schottky defects.
Q: What are some practical applications of Schottky defects?
A: Schottky defects have numerous applications in various fields. They are utilized in solid-state devices, such as transistors and diodes, as well as in catalysts for chemical reactions. Additionally, Schottky defects play a crucial role in determining the properties of ceramic materials and fuel cells.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
