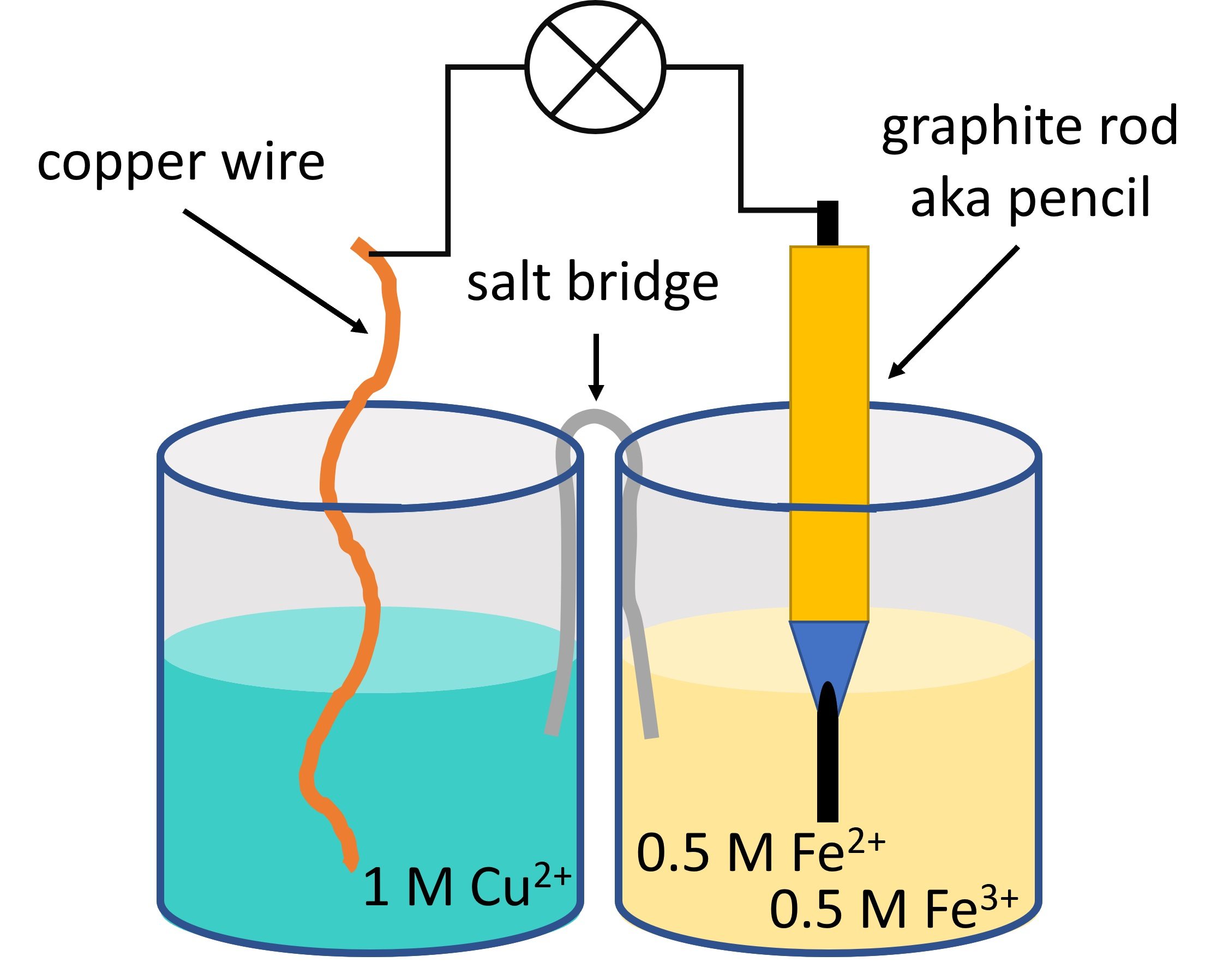
The Nernst equation is a fundamental concept in the field of chemistry that describes the relationship between the concentration of reactants and the potential of an electrochemical cell. Named after the German chemist Walther Nernst, this equation plays a crucial role in understanding and predicting the behavior of redox reactions.
While the Nernst equation may seem complex at first, delving into its intricacies reveals fascinating insights into various chemical processes. In this article, we will explore eight captivating facts about the Nernst equation that highlight its significance and applications. From its derivation to its role in determining cell potential, these facts will provide a deeper understanding of this fundamental equation.
Key Takeaways:
- The Nernst Equation is a super important formula in chemistry that helps scientists understand how electricity works in chemical reactions and biological processes.
- It’s like a secret code that helps scientists figure out the voltage in batteries, the pH of solutions, and even the concentration of substances in different industries.
Nernst Equation is a fundamental equation in electrochemistry.
The Nernst Equation, named after German physicist Walther Hermann Nernst, is a key equation in the field of electrochemistry. It relates the equilibrium potential of a half-cell to the activities or concentrations of the participating species and the temperature. This equation is foundational in studying redox reactions and the behavior of electrochemical cells.
The Nernst Equation is often used to calculate the equilibrium potential of a cell.
By using the Nernst Equation, scientists and chemists can determine the voltage or potential difference between two electrodes in an electrochemical cell when the system is at equilibrium. This calculation allows for the prediction of whether a chemical reaction will proceed spontaneously or not, and it plays a critical role in understanding battery technology and corrosion processes.
The Nernst Equation takes into account temperature and the Faraday constant.
To accurately calculate the equilibrium potential, the Nernst Equation incorporates the temperature of the system and the Faraday constant, which represents the charge on one mole of electrons. The relation between temperature and electrochemical potential is crucial in understanding how temperature affects the spontaneity of a chemical reaction.
The Nernst Equation can be used to determine the pH of a solution.
Through modification and application, the Nernst Equation can also be used to find the pH of a solution. By measuring the potential difference between a reference electrode and a pH electrode, the Nernst Equation can be rearranged to solve for the pH of the solution. This principle is the foundation of pH meters and plays a significant role in chemical analysis and environmental science.
The Nernst Equation has applications in biology and medicine.
The Nernst Equation finds wide-ranging applications in physiological and biochemical systems. It is used to understand and analyze the electrical potential across biological membranes, such as the cell membrane, and the ion concentration gradients that drive nerve impulses, muscle contractions, and other biological processes. The equation is an essential tool in studying the physiology of living organisms and designing medical devices.
The Nernst Equation is a cornerstone in the study of electroanalytical chemistry.
In the field of electroanalytical chemistry, the Nernst Equation is indispensable in the quantitative analysis of various substances. By measuring the potential difference between an indicator electrode and a reference electrode, chemists can determine the concentration of specific ions or molecules in a solution. This technique is widely used in various industries, including environmental monitoring, pharmaceutical analysis, and quality control in manufacturing processes.
The Nernst Equation accounts for the non-ideality of real-world systems.
While the Nernst Equation is derived based on ideal assumptions, it has been modified to incorporate factors that account for non-idealities in real-world systems. These modifications take into consideration activities rather than concentrations, and they consider the impact of ion interactions and deviations from ideal behavior. These adjustments allow for more accurate predictions and calculations in practical applications.
The Nernst Equation has paved the way for advancements in electrochemical research.
Since its development, the Nernst Equation has been instrumental in expanding our understanding of electrochemical phenomena. It has provided researchers with a powerful tool to explore the principles governing electrochemical processes, leading to breakthroughs in energy storage, corrosion prevention, environmental monitoring, and various fields of analytical chemistry. The Nernst Equation continues to shape and inspire further advances in electrochemistry.
Conclusion
The Nernst Equation is a fascinating and fundamental concept in the field of chemistry. By understanding the principles behind this equation, chemists are able to calculate and predict the behavior of electrochemical systems. Here are some key takeaways:1. The Nernst Equation relates the cell potential of an electrochemical cell to the concentration of reactants and products involved.2. It allows us to calculate the equilibrium potential of a cell, providing valuable insights into the underlying chemical reactions.3. The Nernst Equation is pH-dependent, meaning that changes in pH can significantly affect the cell potential.4. It is widely used in various fields, including electrochemistry, biology, and environmental science.5. The equation is named after the German scientist Walther Nernst, who made significant contributions to the field of thermodynamics.6. Nernst Equation is the basis for various experimental techniques, such as potentiometry and ion selective electrode measurements.7. The equation follows the principles of thermodynamics and helps in the understanding of the energy changes occurring in an electrochemical cell.8. Understanding the Nernst Equation is crucial for the design and optimization of electrochemical devices such as batteries and fuel cells.By grasping the concepts underlying the Nernst Equation, scientists and researchers can unlock a deeper understanding of electrochemical processes, opening doors to advancements in various fields.
FAQs
Q: What is the Nernst Equation?
A: The Nernst Equation is an important formula that relates the cell potential of an electrochemical cell to the concentration of reactants and products involved.
Q: Why is the Nernst Equation important?
A: The Nernst Equation allows scientists to calculate the equilibrium potential of a cell and helps in understanding the underlying chemical reactions and energy changes occurring in an electrochemical system.
Q: Can the Nernst Equation be applied to any electrochemical cell?
A: Yes, the Nernst Equation is universally applicable to electrochemical cells, making it a valuable tool in various scientific disciplines.
Q: How does pH affect the Nernst Equation?
A: The Nernst Equation is pH-dependent, meaning that changes in pH can greatly influence the cell potential and the behavior of the electrochemical system.
Q: Who is Walther Nernst?
A: Walther Nernst was a renowned German physical chemist who made significant contributions to thermodynamics and was awarded the Nobel Prize in Chemistry in 1920 for his work on the theoretical and applied aspects of electrochemistry.
Q: How is the Nernst Equation used in practical applications?
A: The Nernst Equation forms the basis for various experimental techniques, such as potentiometry and ion selective electrode measurements, and is instrumental in the development and optimization of electrochemical devices such as batteries and fuel cells.
Exploring the fascinating world of electrochemistry doesn't stop with Nernst's groundbreaking equation. Dive deeper into captivating concepts like redox reactions, which drive countless chemical processes. Uncover the secrets of membrane potentials, essential for understanding cellular communication and ionic gradients. Don't forget to investigate chemical equilibrium, a fundamental principle governing reactions across various fields. Each topic offers a treasure trove of knowledge waiting to be discovered.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
