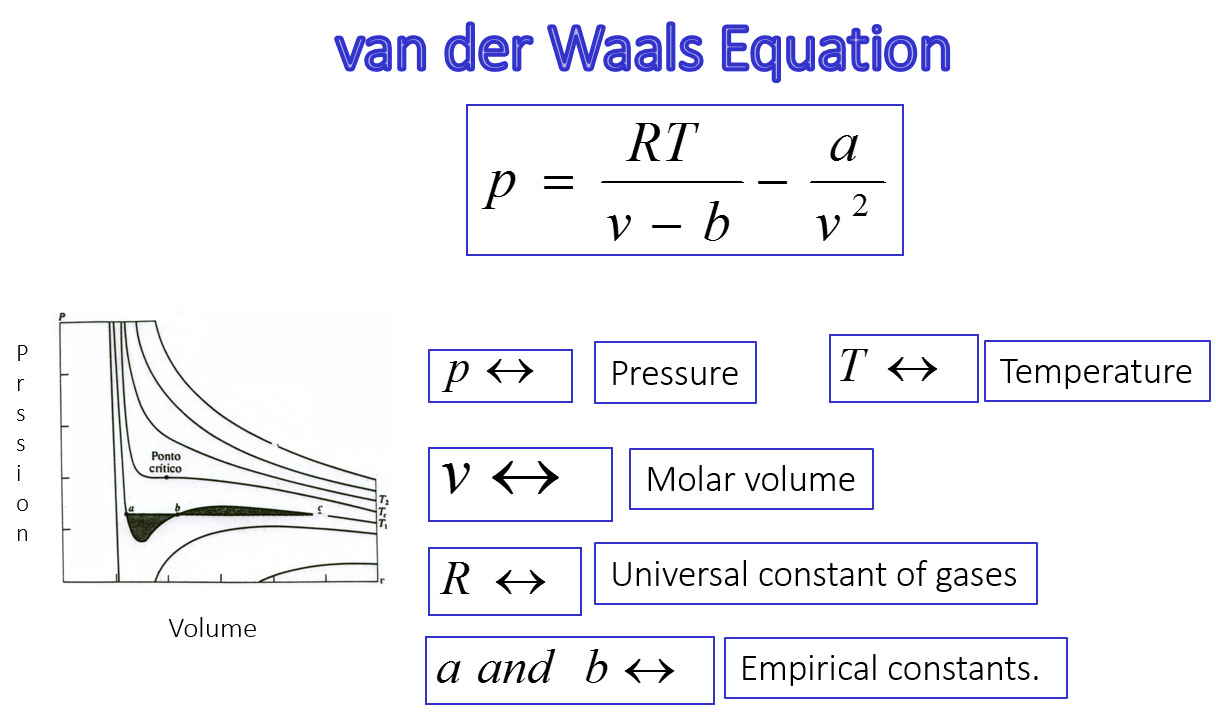
The Van der Waals equation is a fundamental concept in the field of chemistry that describes the behavior of real gases. Developed by Johannes Diderik van der Waals, a Dutch physicist, the equation provides a more accurate representation of gas properties compared to the ideal gas law. While the ideal gas law assumes that gas particles have zero volume and do not interact with each other, the Van der Waals equation takes into account the volume of gas particles and their attractive or repulsive forces.
In this article, we delve into the extraordinary facts surrounding the Van der Waals equation. From its historical significance to its impact on modern chemistry, we explore the key aspects that make it an essential tool in understanding gas behavior. So, let’s dive into the fascinating world of the Van der Waals equation and uncover some intriguing facts about this remarkable scientific breakthrough.
Key Takeaways:
- The Van der Waals equation helps scientists understand how real gases behave, considering factors like particle size and attraction between molecules. It’s like a special formula for gases that don’t behave like we expect them to!
- Johannes Diderik van der Waals, the equation’s creator, won a Nobel Prize for his work. His equation is used in chemistry and engineering to study gases and even predict their critical properties. It’s like a super helpful tool for scientists and engineers!
The Van der Waals equation provides an improved model for real gases.
The Van der Waals equation, formulated by Johannes Diderik van der Waals in 1873, takes into account the non-ideal behavior of gases, considering the intermolecular forces and the finite size of gas particles.
It corrects for the volume occupied by gas particles.
In the Van der Waals equation, the term (V – nb) adjusts for the volume occupied by the gas molecules, where V represents the actual volume and nb represents the volume of the gas particles.
The equation accounts for intermolecular forces.
Unlike the ideal gas law, the Van der Waals equation incorporates the attractive forces between gas molecules. The term (P + an²/V²) corrects for the pressure reduction due to these forces, where P is the observed pressure and an²/V² accounts for the intermolecular attractions.
It accurately predicts the behavior of gases under high pressures.
The Van der Waals equation performs well in predicting the behavior of gases at high pressures, where the attractive forces between particles become significant.
The equation is named after its developer Johannes Diderik van der Waals.
Johannes Diderik van der Waals, a Dutch physicist, formulated the equation to explain the peculiar properties exhibited by real gases.
Van der Waals received the Nobel Prize in Physics for his work.
In 1910, Johannes Diderik van der Waals was awarded the Nobel Prize in Physics for his research on the equation of state for gases and liquids.
It can be used to estimate critical properties of gases.
The Van der Waals equation allows for the calculation of critical temperature, critical pressure, and critical volume, which are essential properties of a substance.
The equation has limitations for gases with high temperatures.
The Van der Waals equation becomes less accurate for gases at very high temperatures, where the effects of intermolecular forces are reduced.
It is commonly used in chemical engineering and thermodynamics.
The Van der Waals equation is widely employed in chemical engineering and thermodynamics to model the behavior of gases and assess deviations from ideal gas behavior.
The equation helps explain the behavior of real gases near their condensation points.
By accounting for intermolecular interactions and particle volume, the Van der Waals equation can shed light on the behavior of gases as they approach their condensation points.
The Van der Waals equation is an improvement over the ideal gas law.
While the ideal gas law assumes that gases are perfectly different from each other and that intermolecular forces and particle volume can be ignored, the Van der Waals equation provides a more accurate representation of real gas behavior.
It is based on the concept of a molecular attraction parameter.
The ‘a’ term in the Van der Waals equation represents the molecular attraction parameter, accounting for the strength of intermolecular forces in a particular gas.
The equation can be derived from a modified form of the ideal gas law.
The Van der Waals equation can be derived from the ideal gas law by introducing additional corrective terms to account for real gas behavior.
It is an equation of state for gases.
The Van der Waals equation provides a mathematical relationship between the pressure, volume, and temperature of real gases, serving as an equation of state.
The Van der Waals equation displays critical behavior.
At temperatures and pressures near the critical point, the Van der Waals equation shows unique characteristics such as a discontinuity in the derivative of the pressure with respect to volume and a point where gas and liquid phases become indistinguishable.
Conclusion
The Van der Waals equation is an remarkable tool used in the field of chemistry to accurately describe the behavior of real gases. It takes into account the intermolecular forces and the finite volume of the gas particles, making it a more accurate representation of gas behavior compared to the ideal gas law. Understanding the Van der Waals equation is crucial for many applications, including predicting phase transitions, determining critical points, and studying the properties of non-ideal gases. By incorporating corrections for intermolecular attractions and particle volume, the Van der Waals equation offers a more comprehensive understanding of gas behavior. Its widespread use in research and industry signifies its importance in the field of chemistry.
FAQs
Q: What is the Van der Waals equation?
A: The Van der Waals equation is an equation of state that describes the behavior of real gases by incorporating corrections for intermolecular attractions and the finite volume of gas particles.
Q: Who proposed the Van der Waals equation?
A: The Van der Waals equation was proposed by Dutch scientist Johannes Diderik van der Waals in 1873.
Q: What is the significance of the Van der Waals equation?
A: The Van der Waals equation is important because it provides a more accurate representation of gas behavior compared to the ideal gas law. It is widely used in various applications, including predicting phase transitions and studying the properties of non-ideal gases.
Q: How does the Van der Waals equation differ from the ideal gas law?
A: The ideal gas law assumes that gas particles have no intermolecular forces and occupy no volume, while the Van der Waals equation incorporates corrections for intermolecular attractions and particle volume.
Q: What are the variables in the Van der Waals equation?
A: The Van der Waals equation includes variables such as pressure, volume, temperature, and constants that represent the intermolecular forces and the finite size of gas particles.
Q: What can the Van der Waals equation be used for?
A: The Van der Waals equation can be used to predict phase transitions, determine critical points, and study the behavior of non-ideal gases. It is an essential tool in the field of chemistry and has various applications in research and industry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


