Gas is a fundamental state of matter that plays a crucial role in various scientific disciplines. While many of us are familiar with the behavior of an ideal gas, it is equally fascinating to explore the world of non-ideal gases. Non-ideal gases exhibit unique properties and behaviors that set them apart from their ideal counterparts. In this article, we will delve into the intriguing world of non-ideal gases and uncover 13 astounding facts that will expand your understanding of the subject. From deviations from the ideal gas law to the influence of intermolecular forces and real-world applications, these facts shed light on the complexities and intricacies of non-ideal gas behavior. So buckle up and get ready to embark on an enlightening journey through the wonders of non-ideal gases.
Key Takeaways:
- Non-ideal gases don’t follow the rules! They behave differently from ideal gases due to intermolecular forces, compressibility, and even phase transitions. It’s like they have a secret life of their own!
- Understanding non-ideal gases is crucial in real-world applications. From chemical engineering to environmental science, knowing how these gases behave in non-ideal conditions is essential for many industries.
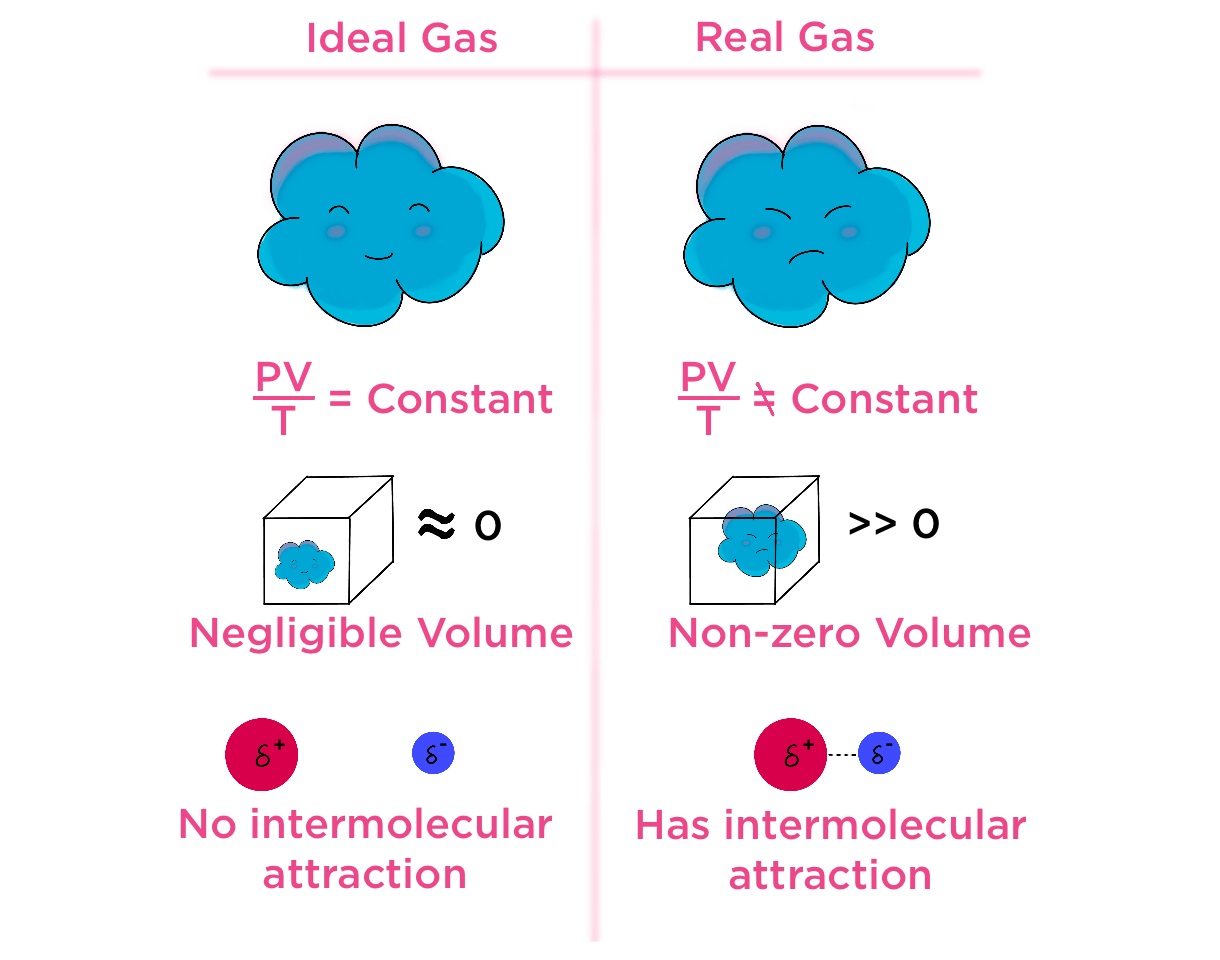
Non-ideal gases deviate from the ideal gas law.
Unlike ideal gases, non-ideal gases do not completely follow the ideal gas law equation PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature.
Intermolecular forces play a significant role.
Non-ideal gases experience intermolecular attractions or repulsions, which affect their behavior and deviate from the simple assumptions made in the ideal gas law.
Non-ideal gases can exhibit compressibility.
Unlike ideal gases, non-ideal gases can be compressed at high pressures due to the intermolecular forces between their particles.
Non-ideal gases may occupy larger volumes than predicted.
Under certain conditions, non-ideal gases occupy larger volumes than expected based on the ideal gas law. This is known as real gas volume.
Non-ideal gases can liquefy at lower temperatures.
Non-ideal gases have a greater tendency to condense and form liquids compared to ideal gases, especially at lower temperatures.
Non-ideal gases exhibit different behavior at high pressures.
At high pressures, non-ideal gases deviate significantly from ideal gas behavior due to the increased impact of intermolecular forces.
Non-ideal gases show variations in heat capacities.
Unlike ideal gases, non-ideal gases have heat capacities that depend on the conditions and intermolecular forces involved.
Non-ideal gases can undergo phase transitions.
Under specific temperature and pressure conditions, non-ideal gases can transition between gas, liquid, and solid states.
Non-ideal gases can exhibit hysteresis.
Hysteresis is observed in non-ideal gases, where the behavior of the gas during expansion is different from that during compression.
Non-ideal gases can form ideal gas mixtures.
While non-ideal gases have deviations from ideal gas behavior individually, when mixed together, they can exhibit ideal gas behavior as a mixture.
Non-ideal gases show variations in diffusion rates.
The diffusion rates of non-ideal gases depend on their molecular mass, temperature, pressure, and the presence of other gases in the mixture.
Non-ideal gases can experience condensation.
Under appropriate conditions, non-ideal gases can undergo condensation and transform into liquids or solids.
Non-ideal gases are extensively studied in real-world applications.
Understanding the behavior of non-ideal gases is crucial in fields such as chemical engineering, environmental science, and industrial processes, where gases are encountered in non-ideal conditions.
These 13 astounding facts about non-ideal gas showcase its complexity and the importance of studying its behavior beyond the ideal gas assumptions. By exploring the interplay of intermolecular forces and deviations from the ideal gas law, scientists delve into the intricacies of different gases and their applications in various industries.
Conclusion
In conclusion, non-ideal gases exhibit fascinating properties that differ from those of ideal gases. From deviations in behavior at high pressures and low temperatures to the formation of intermolecular forces, non-ideal gases have proven to be an intriguing subject of study in the field of chemistry. Understanding the complexities of non-ideal gases is crucial for various applications, such as industrial processes, environmental studies, and even the design of advanced materials.By delving into the 13 astounding facts about non-ideal gases, we have gained valuable insights into their behavior and properties. From the breakdown of the ideal gas law to the impact of intermolecular forces on gas behavior, it is clear that non-ideal gases offer a rich field of research and discovery.As scientists continue to unravel the mysteries of non-ideal gases, it is evident that the study of these substances goes beyond the simple ideal gas model. With a deeper understanding of their unique characteristics, we can apply this knowledge to various areas and propel advancements in chemistry and related fields.
FAQs
Q: What is a non-ideal gas?
A: A non-ideal gas is a gas that does not adhere to the assumptions of the ideal gas law. These gases deviate from ideal behavior due to intermolecular interactions and occupy a larger volume compared to ideal gases under certain conditions.
Q: What causes non-ideal gas behavior?
A: Non-ideal gas behavior is caused by factors such as intermolecular forces, high pressure, and low temperature. These factors influence the behavior and properties of gases, leading to deviations from the ideal gas law.
Q: How do intermolecular forces affect non-ideal gases?
A: Intermolecular forces, such as van der Waals forces, play a significant role in non-ideal gas behavior. These forces cause attractions or repulsions between gas molecules, impacting their interactions and resulting in deviations from ideal gas behavior.
Q: What are some applications of studying non-ideal gases?
A: The study of non-ideal gases has various applications, including industrial processes, environmental studies, and material science. Understanding the behavior of non-ideal gases is crucial for designing efficient chemical processes, predicting atmospheric conditions, and developing advanced materials.
Q: Can non-ideal gases be converted into ideal gases?
A: It is not possible to convert a non-ideal gas into an ideal gas. The behavior of non-ideal gases is inherently different due to factors such as intermolecular forces and deviations from the ideal gas law. However, under certain ideal conditions, non-ideal gases can approximate ideal behavior.
Exploring non-ideal gases reveals fascinating insights into their unique behavior. Dive deeper into the world of molecular interactions by learning about intermolecular forces that shape gas properties. Discover how non-ideal gases undergo phase transitions and explore the critical point on phase diagrams. Uncover the secrets of energy transfer and thermodynamic properties like enthalpy in gases. Embark on a journey through the captivating realm of non-ideal gases and expand your understanding of their complex nature.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


