When it comes to the fascinating world of chemistry, one concept that never fails to capture our attention is the ideal gas. Ideal gases are a fundamental concept in the field of thermodynamics and play a crucial role in explaining the behavior of gases under specific conditions. From classrooms to laboratories, the study of ideal gases provides us with valuable insights into the principles and laws that govern the behavior of matter.
In this article, we will delve deeper into the world of ideal gases and uncover 15 captivating facts that will not only enhance your understanding of this intriguing subject but also ignite your curiosity about the wonders of chemistry. So, fasten your seatbelts and get ready to embark on a fascinating journey exploring the mysterious world of ideal gases!
Key Takeaways:
- Ideal gas is a theoretical concept used in physics and chemistry to simplify calculations and understand gas behavior. While no real gas is truly ideal, the concept is crucial for various applications.
- Ideal gas laws, such as Boyle’s and Charles’s laws, help predict gas behavior. Even though ideal gases don’t exist in reality, they play a vital role in engineering and industry.
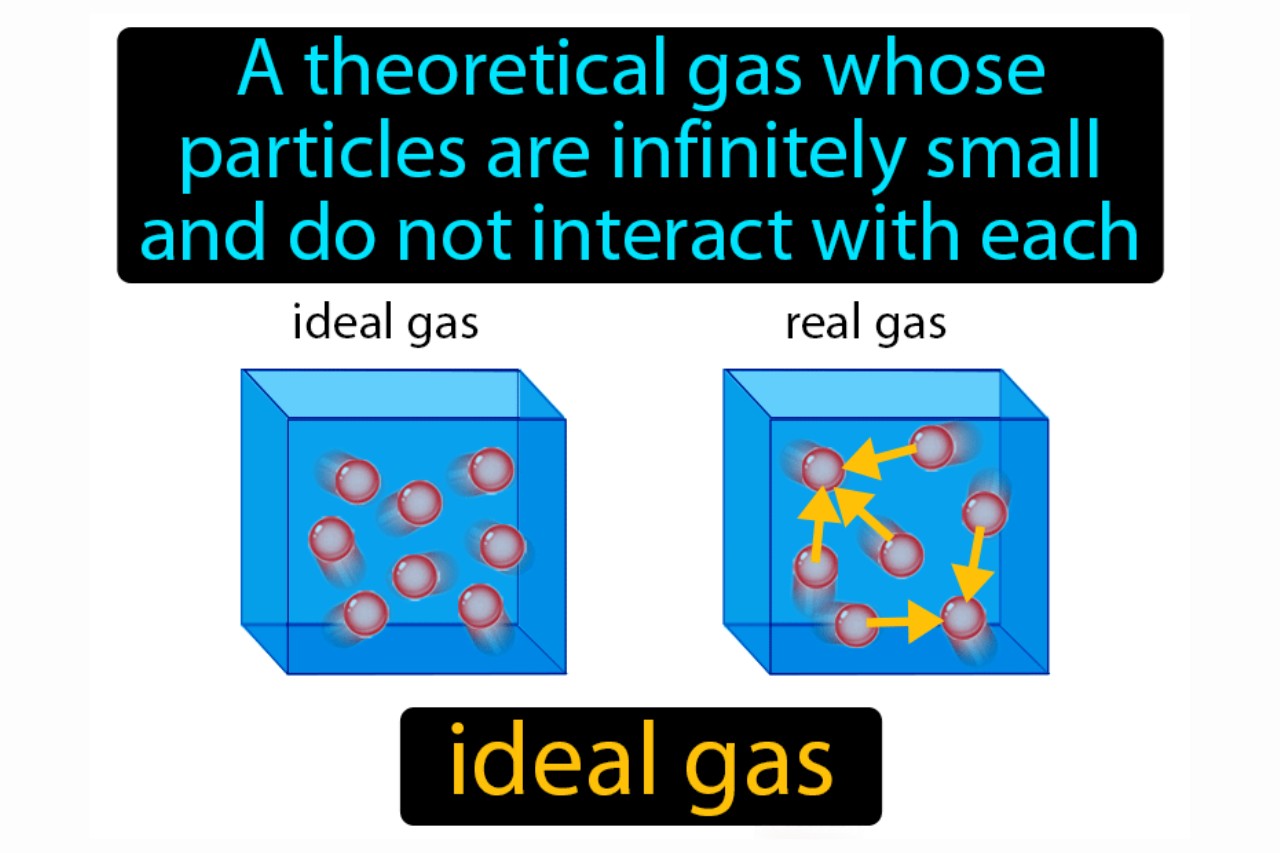
What is an Ideal Gas?
Ideal gas is a theoretical concept in physics and chemistry that describes a gas composed of particles that have negligible volume and do not interact with each other. It is an important concept used to simplify calculations and understand the behavior of gases under idealized conditions.
The Ideal Gas Law
The ideal gas law, expressed as PV = nRT, relates the pressure (P), volume (V), number of moles (n), gas constant (R), and temperature (T) of an ideal gas. This equation is widely used in thermodynamics and is useful in predicting the behavior of gases in various experimental conditions.
Ideal Gases don’t Really Exist
While the concept of an ideal gas is useful for theoretical calculations, no gas in the real world perfectly follows the assumptions of an ideal gas. Real gases deviate from ideal behavior when subjected to high pressures or low temperatures.
The Kinetic Theory of Gases
The kinetic theory of gases explains the behavior of gases, including ideal gases, in terms of the motion of their particles. According to this theory, gas particles are in constant random motion and their kinetic energy is directly proportional to the temperature of the gas.
Boyle’s Law
Boyle’s law states that the volume of an ideal gas is inversely proportional to its pressure, assuming constant temperature. This relationship is expressed as PV = constant.
Charles’s Law
Charles’s law states that the volume of an ideal gas is directly proportional to its temperature, assuming constant pressure. This relationship is expressed as V/T = constant.
Avogadro’s Law
Avogadro’s law states that the volume of an ideal gas is directly proportional to the number of moles of the gas, assuming constant temperature and pressure. This relationship is expressed as V/n = constant.
Combined Gas Law
The combined gas law combines Boyle’s, Charles’s, and Avogadro’s laws into one equation. It allows for the calculation of changes in pressure, volume, and temperature of an ideal gas when all other variables are held constant.
Ideal Gases have No Intermolecular Forces
One of the key assumptions of ideal gases is that the gas particles do not interact with each other. In reality, gases can have intermolecular forces, such as van der Waals forces, which affect their behavior.
Ideal Gases have Uniform Energy Distribution
In an ideal gas, the kinetic energy of the gas particles is uniformly distributed among the particles. This distribution is known as the Maxwell-Boltzmann distribution.
Ideal Gases Follow the Laws of Thermodynamics
Even though ideal gases do not exist in reality, they follow the laws of thermodynamics. These laws describe the relationships between temperature, energy, entropy, and other properties of gases.
Real Gases Approach Ideal Behavior under Certain Conditions
Although real gases deviate from ideal behavior, they can approximate ideal gas behavior under certain conditions of low pressure and high temperature. This is known as the ideal gas limit.
The Ideal Gas Law Applies to Mixtures of Gases
The ideal gas law can be applied to mixtures of gases by considering the total pressure, total volume, and total number of moles of the mixture. Each gas in the mixture acts as if it were the only gas present.
Ideal Gas Behavior is Assumed in Many Chemical Reactions
In many chemical reactions, gases are treated as ideal to simplify calculations and predictions. This assumption allows for easier analysis of reaction stoichiometry and other thermodynamic properties.
Ideal Gases Play a Crucial Role in Engineering and Industry
Ideal gas concepts are widely used in engineering and industry for designing and analyzing systems involving gases. From air conditioning and refrigeration to power generation and chemical processes, ideal gas behavior is an essential consideration.
Conclusion
In conclusion, ideal gases are a fascinating topic in chemistry that have a range of captivating facts. From their behavior under certain conditions to their relationship with the ideal gas law, ideal gases offer insights into the fundamental principles of chemistry. Whether you’re a chemistry enthusiast or just curious about the world around you, understanding ideal gases can help deepen your knowledge of how matter behaves.
By studying ideal gases, scientists and researchers have been able to develop applications in various fields, such as engineering, environmental science, and even everyday life. The concept of ideal gases simplifies complex systems and allows for convenient calculations and predictions. Understanding the properties of ideal gases is crucial in many scientific and industrial processes.
So, the next time you hear the term “ideal gas,” remember the fascinating facts that make them so intriguing. From their hypothetical nature to their role in understanding real-world gases, ideal gases are a cornerstone of chemical knowledge.
FAQs
Q: What is an ideal gas?
A: An ideal gas is a hypothetical gas that follows the ideal gas law, which describes the relationship between its pressure, volume, temperature, and number of particles.
Q: Are real gases considered ideal?
A: Real gases do not perfectly adhere to the ideal gas law. However, under certain conditions, real gases can behave like ideal gases and exhibit similar properties.
Q: What is the ideal gas law?
A: The ideal gas law, represented by the equation PV = nRT, relates the pressure (P), volume (V), number of gas particles (n), gas constant (R), and temperature (T) for an ideal gas.
Q: Can an ideal gas be liquefied?
A: No, an ideal gas cannot be liquefied as it is a hypothetical concept that assumes particles do not interact with each other. Real gases can be liquefied through processes such as cooling and increasing pressure.
Q: Is air considered an ideal gas?
A: Air is not considered an ideal gas as it consists of a mixture of various gases and contains impurities. However, at certain conditions and within a limited range, air can exhibit behavior similar to an ideal gas.
Q: Why are ideal gases important in chemistry?
A: Ideal gases serve as a simplified model system that allows scientists to understand and predict the behavior of real gases in different conditions. They are crucial for studying and formulating scientific principles in various fields of chemistry and related disciplines.
Ideal gases captivate chemists, but there's more to explore! Dive deeper into ideal gas law's extraordinary facts and astounding insights about nonideal gases. Satisfy your curiosity and expand knowledge beyond these 15 fascinating tidbits. Uncover additional mind-blowing revelations that'll leave you amazed at gases' behavior under various conditions. Chemistry enthusiasts won't want to miss out on this treasure trove of information!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


