The Joule-Thomson law of gases is a fundamental principle in physics that helps us understand the behavior of gases under various conditions. Named after James Prescott Joule and William Thomson (also known as Lord Kelvin), this law provides valuable insights into the cooling or heating effect that occurs when a gas expands or is compressed at a constant temperature. Understanding the Joule-Thomson law is crucial in many scientific and technological applications, including refrigeration, liquefaction of gases, and the study of thermodynamics. In this article, we will delve into this fascinating law and uncover 18 intriguing facts that will broaden your understanding of its principles and applications. So, get ready to embark on a journey through the fascinating world of the Joule-Thomson law of gases!
Key Takeaways:
- The Joule-Thomson effect explains how gases cool or heat up when they expand or are compressed, impacting industries from natural gas to medical treatments.
- Different gases behave uniquely under pressure changes, allowing scientists and engineers to predict and control temperature changes for various applications.
The Joule-Thomson Effect
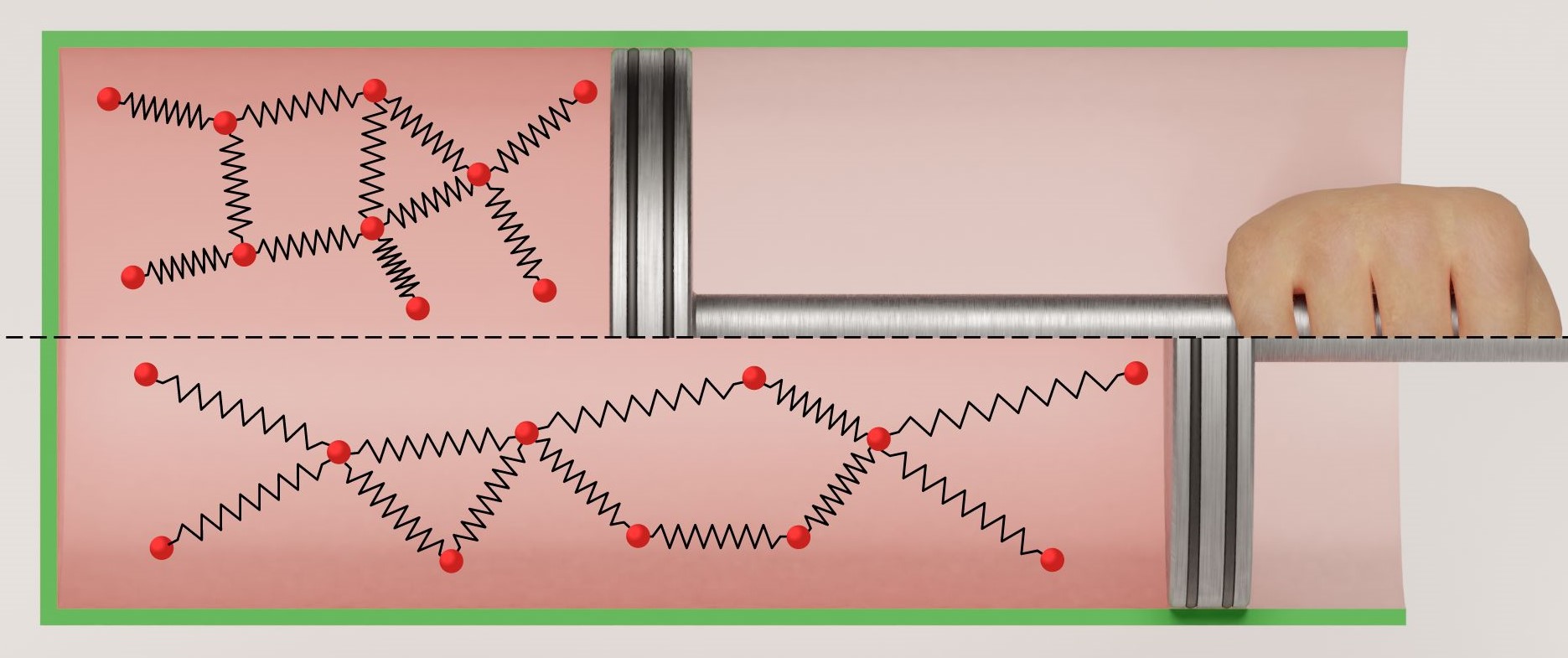
The Joule-Thomson effect, also known as the Joule-Kelvin effect, is the phenomenon where the temperature of a gas changes when it undergoes a throttling process.
Description of the Law
The Joule-Thomson law states that when a gas expands into a region of lower pressure, it experiences a temperature change. If the gas experiences a decrease in temperature, it is said to exhibit a “Joule-Thomson cooling effect.” Conversely, if the gas undergoes an increase in temperature, it is referred to as a “Joule-Thomson heating effect.”
Application in the Liquefaction of Gases
The Joule-Thomson effect plays a vital role in the liquefaction of gases. By subjecting a gas to high pressure and then allowing it to expand through a small opening, the temperature of the gas can be reduced significantly, leading to its liquefaction.
Inverse Relationship Between Temperature and Pressure
According to the Joule-Thomson law, there is an inverse relationship between the temperature and pressure change. If the gas undergoes a pressure decrease, it will experience a temperature increase, and vice versa.
Gas Behavior Predictions
By applying the Joule-Thomson law, scientists can predict the behavior of gases under various conditions, such as expansion, compression, and cooling.
Positive and Negative Joule-Thomson Coefficient
A positive Joule-Thomson coefficient indicates that the gas cools upon expansion, while a negative coefficient signifies heating upon expansion.
Influence of Gas Properties
The Joule-Thomson effect is influenced by the nature of the gas itself. Different gases exhibit unique behaviors, leading to varying temperature changes upon expansion.
Real Gas Deviations
Real gases may deviate from ideal behavior when undergoing the Joule-Thomson process, as intermolecular forces come into play.
Cooling Effect of Carbon Dioxide
Carbon dioxide displays a significant cooling effect when subjected to the Joule-Thomson process, making it essential in industrial refrigeration applications.
Experimental Validation
The Joule-Thomson effect was experimentally validated by James Prescott Joule and William Thomson (Lord Kelvin) in the mid-19th century through meticulous observations and measurements.
Importance in Natural Gas Industry
The Joule-Thomson effect is crucial in the natural gas industry for processes like natural gas processing, purification, and transportation.
Medical Applications
The Joule-Thomson effect has medical applications, such as in cryotherapy, where controlled cooling is used for therapeutic purposes.
Negative Temperature Change
The Joule-Thomson effect can lead to a negative temperature change, cooling gases to sub-zero temperatures, including the point of liquefaction.
Engineering Considerations
Engineers must consider the Joule-Thomson effect when designing systems involving gas flow and pressure changes to ensure efficient and effective operations.
Study of Phase Transitions
The Joule-Thomson effect contributes to the study of phase transitions, particularly the changes between gas and liquid states.
Impact on Gas Pipelines
Joule-Thomson cooling can affect the efficiency of natural gas pipelines, as the reduction in temperature can cause the gas to condense, resulting in pressure drops and potential pipeline blockages.
Calculation of Inversion Temperature
The inversion temperature is when the Joule-Thomson coefficient becomes zero. It is a critical value in the analysis of gas behavior under various conditions.
Understanding Gas Behavior
The Joule-Thomson effect provides valuable insights into the behavior of gases and contributes to our understanding of thermodynamics and heat transfer.
This comprehensive guide presented 18 intriguing facts about the Joule-Thomson Law of Gases. From its application in the liquefaction of gases to its influence in the natural gas industry, understanding the Joule-Thomson effect is essential in various scientific and engineering fields. By grasping the unique behaviors of different gases under pressure changes, we can harness this knowledge for applications ranging from industrial refrigeration to medical treatments. The Joule-Thomson law enables us to predict and control temperature changes, shedding light on the fascinating world of thermodynamics and gas behavior.
Conclusion
In conclusion, the Joule-Thomson law of gases is a fascinating concept in physics that plays a crucial role in understanding the behavior of gases under changing conditions of pressure and temperature. Through this law, scientists have been able to unravel the intricacies of gas dynamics and develop practical applications in various fields, including refrigeration technology and natural gas processing.With its origins dating back to the 19th century, the Joule-Thomson law has stood the test of time and continues to be a cornerstone in the study of thermodynamics. It demonstrates how the internal energy of gases can be affected by changes in enthalpy and entropy, leading to the phenomenon of cooling or heating when gases expand or contract. This understanding has paved the way for advancements in energy-efficient cooling systems and cryogenic technologies.From the surprising effects on the cooling of compressed gases to the intriguing inversion temperature phenomenon, the Joule-Thomson law offers a rich source of scientific discoveries and practical applications. By delving into the intricacies of this law, scientists and engineers continue to unlock new possibilities for improving technological processes and expanding our knowledge of the behavior of gases.Ultimately, the Joule-Thomson law reminds us of the remarkable connections between the fundamental principles of physics and their tangible impact on our daily lives. Whether it’s enjoying the benefits of a well-functioning refrigeration system or exploring the frontiers of cryogenics, the fascination and utility of the Joule-Thomson law are certain to endure for years to come.
FAQs
1. What is the Joule-Thomson law of gases?
The Joule-Thomson law of gases states that under certain conditions, the temperature of a gas can change when it is allowed to expand or contract without the exchange of heat with its surroundings.
2. What is the significance of the Joule-Thomson law?
The Joule-Thomson law is significant as it helps us understand and predict how gases behave when subjected to changes in pressure and temperature. It has practical applications in fields such as refrigeration, natural gas processing, and cryogenics.
3. What is the Joule-Thomson coefficient?
The Joule-Thomson coefficient is a measure of how much the temperature of a gas changes for a given change in pressure, keeping all other variables constant.
4. What is the inversion temperature in the context of the Joule-Thomson effect?
The inversion temperature is the temperature at which there is no temperature change when a gas undergoes the Joule-Thomson expansion or compression process. Below this temperature, the gas cools upon expansion, and above this temperature, the gas heats up upon expansion.
5. How is the Joule-Thomson effect utilized in refrigeration?
The Joule-Thomson effect is crucial in the operation of refrigerators and air conditioners. By expanding a refrigerant gas using the Joule-Thomson effect, its temperature decreases, enabling it to absorb heat from the surroundings, thus cooling the desired space.
Exploring the fascinating world of thermodynamics and gas behavior doesn't stop with Joule-Thomson law. Dive deeper into physical chemistry principles, uncover mind-blowing Joule-Thomson effect facts, or discover extraordinary insights about enthalpy. Each topic offers a unique perspective on the complex interactions between matter and energy, providing a comprehensive understanding of the fundamental laws governing our universe. Whether you're a curious learner or a seasoned scientist, these captivating articles will expand your knowledge and ignite your passion for the incredible world of thermodynamics.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.