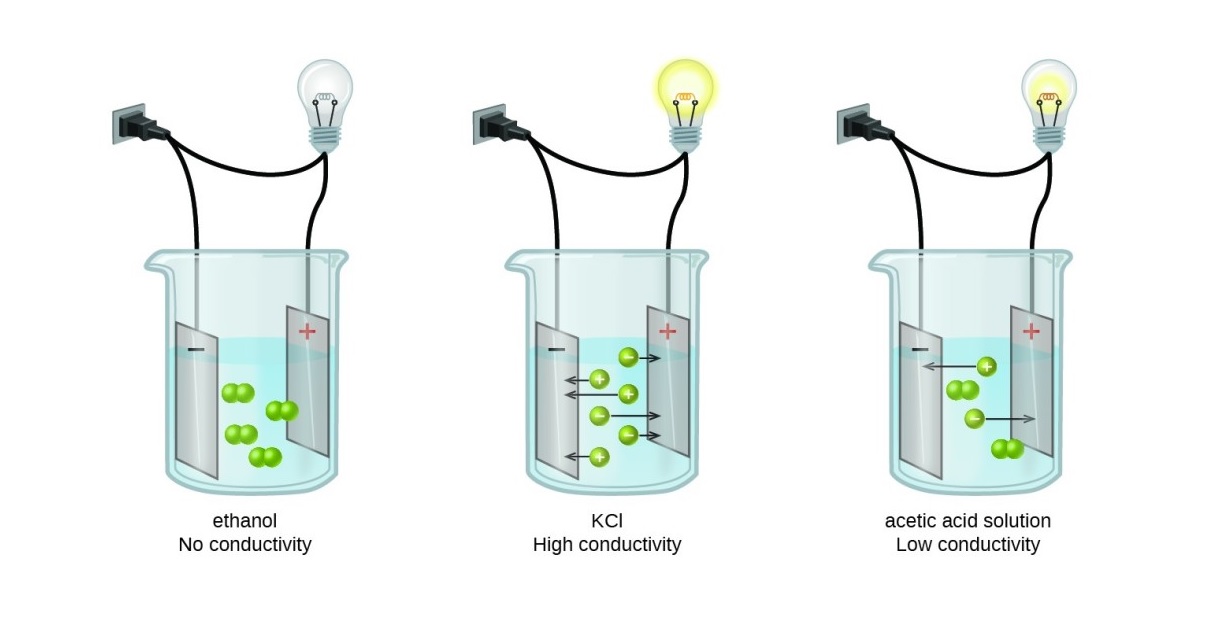
Electrolytic conductivity is a fascinating concept in the world of chemistry. It plays a vital role in understanding the behavior of substances and their ability to conduct electricity. Whether you’re a chemistry enthusiast or just curious about the subject, delving into the realm of electrolytic conductivity can be both intriguing and educational.
In this article, we will explore ten intriguing facts about electrolytic conductivity that will not only expand your knowledge but also ignite your curiosity. From the fundamentals of electrolytes to the factors affecting conductivity, we will unravel the mysteries behind this essential concept.
So fasten your seatbelt as we journey through the realm of electrolytic conductivity and discover some fascinating facts that will leave you electrified with awe and wonder!
Key Takeaways:
- Electrolytic conductivity is the ability of substances to conduct electricity when dissolved in water, and it’s super important in science and industry.
- Factors like ion concentration and temperature affect electrolytic conductivity, which is crucial for processes like electroplating and assessing water quality.
What is Electrolytic Conductivity?
Electrolytic conductivity refers to the ability of a substance to conduct electricity when dissolved in a solvent, usually water. This property is essential in many scientific and industrial applications.
Importance of Electrolytic Conductivity
Electrolytic conductivity plays a crucial role in various fields such as chemistry, biology, and engineering. It helps in understanding the behavior of electrolytes and their impact on solutions and systems.
Factors Affecting Electrolytic Conductivity
Several factors influence the conductivity of electrolytes, including the concentration of ions, temperature, and the nature of the solvent. These factors can significantly alter the conductivity of a solution.
Applications in Electrochemistry
Electrolytic conductivity is extensively used in electrochemical processes such as electroplating, electrolysis, and fuel cell technology. It enables the efficient transfer of ions and facilitates the conversion of chemical energy into electrical energy.
Conductivity Measurement
The conductivity of a substance can be measured using a conductivity meter, which determines the ability of the substance to carry an electrical current. This measurement is crucial for assessing the quality of water, monitoring industrial processes, and ensuring product safety.
Conductivity and Salinity
There is a direct relationship between electrolytic conductivity and the salinity of a solution. Higher concentrations of dissolved ions, such as salts, result in increased conductivity.
Conductivity in Biological Systems
Electrolytic conductivity is essential for the proper functioning of biological systems, including the human body. It facilitates the transmission of nerve impulses and aids in maintaining the balance of electrolytes and hydration levels.
Electrolytic Conductivity of Metals
Metallic substances are known for their high electrical conductivity. However, when metals are dissolved in a liquid or transformed into an ionic state, their electrolytic conductivity can vary significantly.
Relationship with pH
The pH of a solution can influence its conductivity. In general, solutions with higher acidity or alkalinity tend to exhibit higher conductivity due to the presence of more ions.
Electrolytic Conductivity and Water Quality
Measurements of electrolytic conductivity are commonly used to assess the quality of water. It helps in identifying impurities, contaminants, and the overall health of aquatic ecosystems. High conductivity levels may indicate the presence of pollutants.
Conclusion
Electrolytic conductivity is a fascinating concept that plays a crucial role in various fields, including chemistry, materials science, and electrical engineering. In this article, we explored 10 intriguing facts about electrolytic conductivity that shed light on the behavior of electrolytes and their ability to conduct electric currents.
We learned that electrolytic conductivity is influenced by factors such as temperature, concentration of ions, and the nature of the solvent. We also discovered that different compounds and substances exhibit varying levels of conductivity due to their unique molecular structures.
Understanding electrolytic conductivity is essential for developing and optimizing technologies like batteries, fuel cells, and electrochemical sensors. By harnessing the principles of electrolytic conductivity, researchers and engineers can create more efficient and reliable devices that power our modern world.
As our knowledge of electrolytic conductivity continues to expand, so does our ability to design and innovate in fields that rely on electrical conductivity. By delving deeper into this intricate concept, we unlock new possibilities for advancements in science and technology.
FAQs
Q: What is electrolytic conductivity?
A: Electrolytic conductivity refers to the ability of a substance or solution to conduct an electric current. It is a property that arises from the movement of charged particles (ions) within the substance or solution.
Q: What factors affect electrolytic conductivity?
A: Several factors influence electrolytic conductivity, including temperature, concentration of ions, and the nature of the solvent. Increasing the temperature generally enhances conductivity, while increasing ion concentration also leads to higher conductivity.
Q: What are some examples of highly conductive electrolytes?
A: Common examples of highly conductive electrolytes include acids, bases, salts, and certain ions in aqueous solutions. These substances contain freely moving ions that enable the passage of electric current.
Q: How is electrolytic conductivity measured?
A: Electrolytic conductivity is measured using a device called a conductivity meter. This instrument measures the ability of a solution or substance to conduct an electric current by determining the resistance encountered by the current as it passes through the sample.
Q: What are the practical applications of electrolytic conductivity?
A: Electrolytic conductivity has many practical applications, including battery technology, fuel cells, electroplating, water purification, and chemical analysis. It is also crucial in industries such as electronics, pharmaceuticals, and environmental monitoring.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
