Kohlrausch’s Law is a fundamental concept in the field of electrochemistry, which describes the behavior of electrolyte solutions. Named after Friedrich Kohlrausch, a German physicist and chemist, this law has revolutionized our understanding of how ions conduct electricity in solutions.
In this article, we will delve into the fascinating world of Kohlrausch’s Law and explore twenty unbelievable facts that will leave you awestruck. From its historical background to its applications in various scientific disciplines, we will uncover the intricate details and practical implications of this law.
So, fasten your seatbelts and get ready for an exhilarating journey into the world of Kohlrausch’s Law, where we will unravel the mysteries and unveil the amazing insights that this scientific principle has to offer.
Key Takeaways:
- Kohlrausch’s Law explains how ions in a solution affect its conductivity, helping scientists understand and improve processes like water treatment and energy conversion.
- This fundamental law by Friedrich Kohlrausch has wide-ranging applications in fields like chemistry, biochemistry, and materials science, contributing to advancements in various industries.
Kohlrausch’s Law is named after the German physicist Friedrich Kohlrausch.
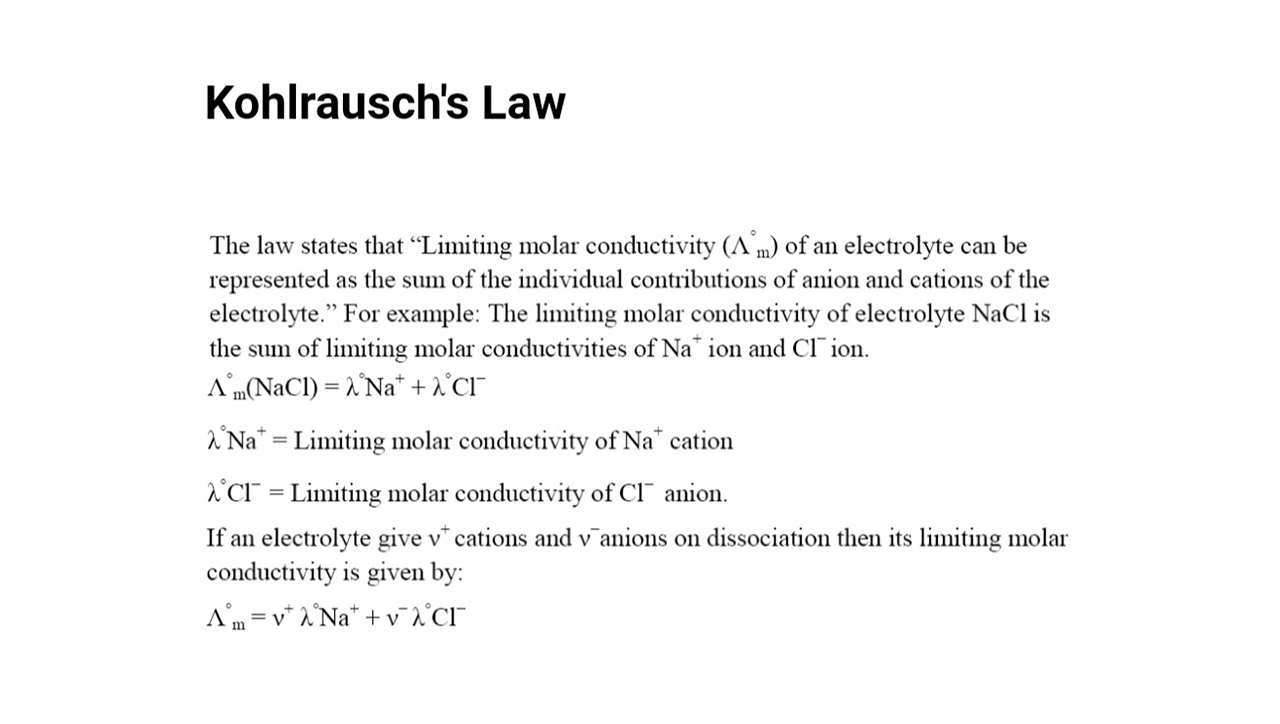
Kohlrausch’s Law, also known as the Law of Independent Migration of Ions, was first formulated by Friedrich Kohlrausch in the late 19th century.
Kohlrausch’s Law is a fundamental concept in electrochemistry.
This law describes the behavior of electrolytes, which are substances that conduct electricity when dissolved in a solvent, such as water.
Kohlrausch’s Law states that the conductivity of an electrolyte solution is directly proportional to the concentration of ions.
According to this law, the more ions present in a solution, the higher its conductivity will be.
Kohlrausch’s Law is applicable to various types of electrolytes.
Whether it is a strong electrolyte, weak electrolyte, or even a mixture of electrolytes, this law holds true.
Kohlrausch’s Law plays a crucial role in determining the transport properties of electrolytes.
It helps in understanding how ions move and interact in solution, which is essential for various industrial and scientific applications.
Kohlrausch’s Law can be used to determine the degree of dissociation of weak electrolytes.
By measuring the conductivity of solutions containing weak electrolytes, one can calculate the extent to which they dissociate into ions.
Kohlrausch’s Law has applications in fields such as chemistry, biochemistry, and materials science.
It is particularly useful in studying the behavior of electrolytes in biological systems and in the development of new materials with desired properties.
Kohlrausch’s Law helps in the characterization of ionic solutions.
By measuring the conductivity of solutions at different concentrations and temperatures, scientists can gather valuable information about the nature and behavior of ions.
Kohlrausch’s Law is based on the concept of ionic mobility.
Ionic mobility refers to the ability of ions to move in a solution under the influence of an electric field.
Kohlrausch’s Law allows the calculation of equivalent conductance.
Equivalent conductance is a measure of the conductivity of a solution containing one equivalent of an electrolyte.
Kohlrausch’s Law is a cornerstone in the study of conductometric titrations.
Conductometric titrations involve using conductivity measurements to determine the endpoint of a chemical reaction.
Kohlrausch’s Law helps in understanding the properties of solutions at high dilutions.
At high dilutions, ionic interactions become less significant, and the behavior of electrolytes can be better described by Kohlrausch’s Law.
Kohlrausch’s Law is essential for the design and optimization of fuel cells.
It aids in the development of efficient and sustainable energy conversion devices by understanding the behavior of ions in the electrolyte solution.
Kohlrausch’s Law provides a theoretical foundation for the measurement of conductivity in laboratories.
It helps scientists accurately measure the conductivity of various solutions, providing valuable data for research and analysis.
Kohlrausch’s Law has contributed to advancements in water treatment and purification processes.
Understanding the behavior of ions in solution has led to improved techniques for removing contaminants from water sources.
Kohlrausch’s Law has opened doors for the development of sensors and detectors based on ionic conductivity.
These devices play a vital role in the detection of various analytes, such as pH, ions, and biomolecules.
Kohlrausch’s Law helps in the study of electrolyte solutions under different conditions, such as temperature and pressure.
By studying how conductivity changes with varying conditions, scientists can gain insights into the behavior of electrolytes in diverse environments.
Kohlrausch’s Law has been verified and validated by numerous experimental studies.
Scientists have conducted extensive research to confirm the accuracy and reliability of this fundamental law in various experimental setups.
Kohlrausch’s Law is a testament to the importance of quantitative measurements in the field of chemistry.
By providing a mathematical relationship between conductivity and ion concentration, this law highlights the significance of precise measurements in scientific investigations.
Kohlrausch’s Law continues to be a topic of research and exploration in modern-day science.
Scientists are continually expanding their understanding of electrolytes and how they behave, further enhancing the applications and significance of Kohlrausch’s Law.
Conclusion
In conclusion, Kohlrausch’s Law is a fundamental concept in the field of chemistry that helps us understand the conductivity of electrolyte solutions. Through this law, we have uncovered some truly astonishing facts that expand our knowledge of the behavior of ions in solution.From the correlation between ionic conductivity and concentration to the prediction of unknown concentrations using empirical data, Kohlrausch’s Law has proven to be a powerful tool. It has provided scientists with a deeper understanding of the principles behind electrolysis, conductivity measurements, and the behavior of electrolytes in various settings.As we continue to explore the intricacies of chemistry, Kohlrausch’s Law will undoubtedly remain a cornerstone in our quest to unravel the mysteries of electrolyte solutions. Its wide range of applications and its ability to predict and analyze conductivity make it an indispensable concept for chemists and researchers alike.By delving into the fascinating facts surrounding Kohlrausch’s Law, we not only broaden our understanding of the field of chemistry but also gain a deeper appreciation for the remarkable intricacies that govern the behavior of chemicals and their interactions in solution.
FAQs
1. What is Kohlrausch’s Law?
Kohlrausch’s Law states that the molar conductivity of an electrolyte solution at infinite dilution is the sum of the molar conductivities of its constituent ions.
2. What is the significance of Kohlrausch’s Law?
Kohlrausch’s Law is essential in understanding the behavior of electrolyte solutions and predicting their conductivity. It provides insight into the dissociation of ions and allows for the determination of unknown concentrations in solutions.
3. How is Kohlrausch’s Law applied in practice?
Kohlrausch’s Law is applied through conductivity measurements of electrolyte solutions at various concentrations. The obtained data can be used to calculate the molar conductivities of individual ions, helping in the analysis of solutions and predicting conductivity at infinite dilution.
4. Can Kohlrausch’s Law be applied to all types of electrolytes?
Yes, Kohlrausch’s Law is applicable to all types of electrolytes, including strong electrolytes that fully dissociate into ions and weak electrolytes that only partially dissociate. However, the accuracy of the law’s predictions may vary depending on the degree of dissociation.
5. How does Kohlrausch’s Law relate to other laws in chemistry?
Kohlrausch’s Law is closely related to Ohm’s Law and Faraday’s Laws of electrolysis. While Ohm’s Law relates current, voltage, and resistance, Kohlrausch’s Law focuses specifically on the ionic conductivity of solutions. Faraday’s Laws, on the other hand, explain the relation between the amount of substance involved in electrolysis and the electric current.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
