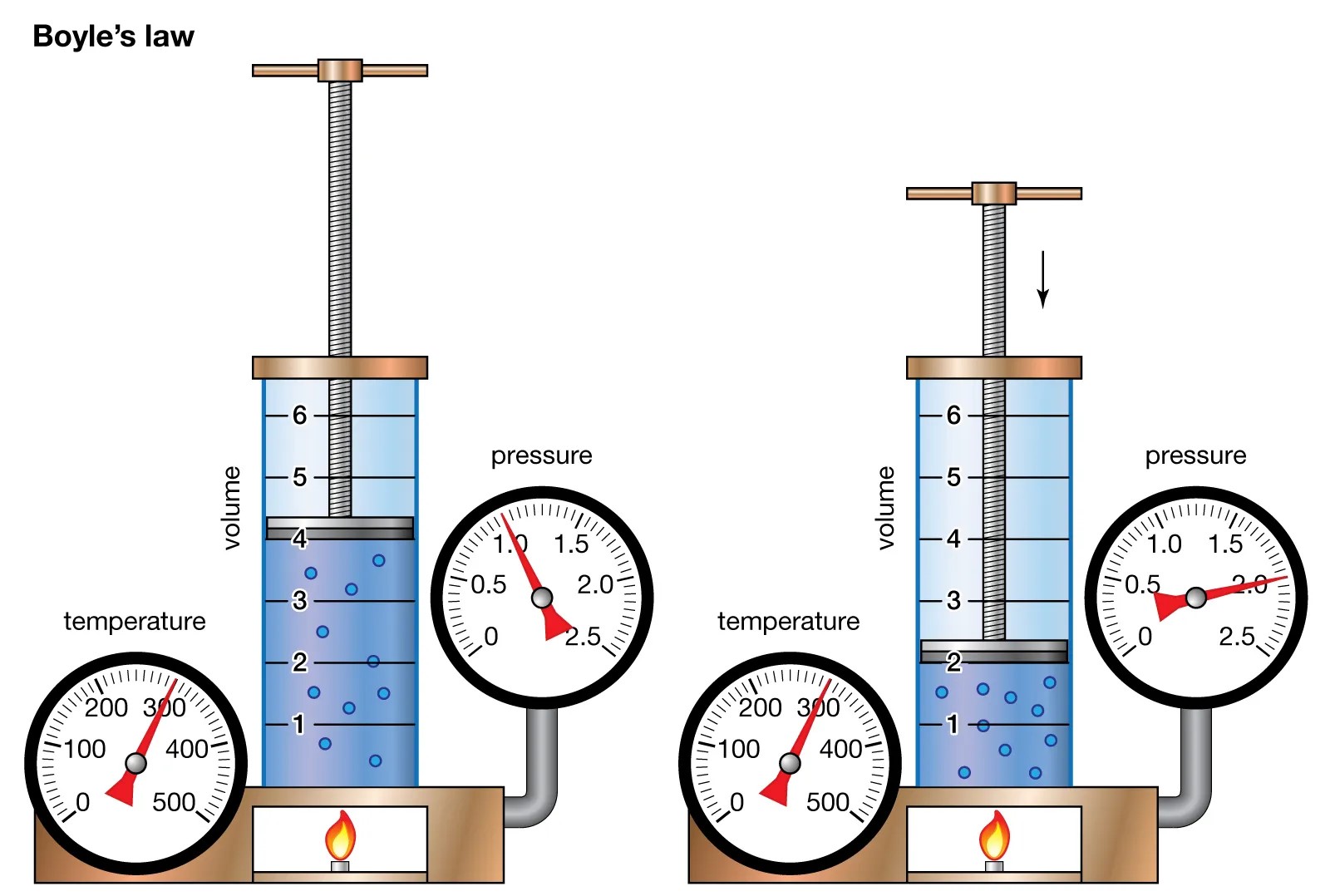
The study of physics encompasses a wide range of fascinating laws and principles that govern the behavior of matter and energy. One of these fundamental laws is Boyle’s Law, which explores the relationship between the pressure and volume of a gas at constant temperature.
Boyle’s Law, named after the Irish scientist Robert Boyle, was discovered in the 17th century and has revolutionized our understanding of how gases behave. It forms the basis for many practical applications in fields such as engineering, chemistry, and even medicine.
In this article, we will delve deeper into Boyle’s Law and uncover 14 unbelievable facts that will leave you awestruck. From mind-boggling experiments to mind-blowing applications, get ready to explore the wonders of Boyle’s Law and its implications on our everyday lives.
Key Takeaways:
- Boyle’s Law explains how gas pressure and volume are related. It’s like a seesaw: when one goes up, the other goes down. This law helps scuba divers and engineers in their work.
- Boyle’s Law, discovered by scientist Robert Boyle, is crucial in understanding gases and their behavior. It’s like a secret code that helps scientists and engineers in many different fields.
Boyle’s Law describes the relationship between the pressure and volume of a gas.
Boyle’s Law, named after scientist Robert Boyle, states that the pressure of a gas is inversely proportional to its volume when temperature is kept constant. In simple terms, as the volume of a gas decreases, its pressure increases, and vice versa.
Boyle’s Law is expressed mathematically as P1V1 = P2V2.
This equation represents the initial and final states of a gas. P1 and V1 represent the initial pressure and volume, while P2 and V2 represent the final pressure and volume. It shows that the product of pressure and volume remains constant as long as the temperature does not change.
Boyle’s Law is applicable to both ideal and real gases.
Whether it’s an ideal gas that follows the ideal gas law or a real gas with intermolecular forces, Boyle’s Law holds true for both. It is a fundamental principle in the study of gases and plays a crucial role in various applications.
Boyle’s Law is an essential concept in scuba diving.
Scuba divers rely on Boyle’s Law to understand the changes in pressure and volume as they descend or ascend in the water. By monitoring their air supply and adjusting their equipment accordingly, divers can ensure their safety underwater.
Boyle’s Law helps explain the functioning of respiratory systems.
The mechanism of breathing can be explained using Boyle’s Law. During inhalation, the diaphragm and intercostal muscles expand the thoracic cavity, decreasing its pressure and allowing air to rush into the lungs. On the contrary, during exhalation, the volume decreases, increasing the pressure and forcing air out of the lungs.
Boyle’s Law is used in the design and operation of compressed air systems.
From industrial machinery to scuba diving equipment, compressed air systems are widely used. Applying Boyle’s Law helps engineers and operators understand the relationship between pressure, volume, and temperature when working with compressed gases.
The inverse relationship described by Boyle’s Law is graphically represented by a hyperbola.
A graph of pressure versus volume under constant temperature conditions forms a hyperbolic curve, showcasing the inverse relationship between the two variables. As one increases, the other decreases, creating a distinct shape on the graph.
Boyle’s Law is a fundamental concept in the field of thermodynamics.
Thermodynamics, the study of energy and its transformations, heavily relies on Boyle’s Law. It is one of the key principles used to understand and analyze the behavior of gases in various energy-related processes.
Boyle’s Law can be explained using particle theory.
According to the particle theory, as the volume of a container decreases, the gas particles inside have less space to move, resulting in a higher frequency of collisions with the walls of the container. This increased collision frequency leads to a higher pressure.
Boyle’s Law is named after Irish scientist Robert Boyle.
Robert Boyle, an influential physicist and chemist, discovered the relationship between pressure and volume in the mid-17th century. His experiments and observations formed the basis of what is now known as Boyle’s Law.
Boyle’s Law is one of the gas laws along with Charles’ Law and Gay-Lussac’s Law.
Boyle’s Law, Charles’ Law, and Gay-Lussac’s Law are three fundamental gas laws that describe the behavior of gases under different conditions. These laws collectively provide a comprehensive understanding of gas properties.
Boyle’s Law is an example of an inverse relationship.
An inverse relationship means that as one variable increases, the other variable decreases, and vice versa. In the case of Boyle’s Law, an increase in pressure leads to a decrease in volume, and a decrease in pressure leads to an increase in volume.
Boyle’s Law is a foundational concept in the study of fluid dynamics.
Fluid dynamics, the study of how fluids behave and interact with forces, relies on understanding the principles of Boyle’s Law. It helps explain the behavior of gases and liquids in various scenarios.
Boyle’s Law is applicable to a wide range of fields, including chemistry, physics, and engineering.
Due to its versatile nature, Boyle’s Law finds applications in numerous scientific and engineering disciplines. Its principles are utilized in areas such as chemical reactions, gas dynamics, and even the functioning of everyday household appliances.
Conclusion
Boyle’s Law is a fundamental principle in physics that provides insights into the behavior of gases. By understanding the relationship between pressure and volume, we can make predictions about how changes in one variable affect the other. The astonishing facts about Boyle’s Law shed light on its significance and application in various fields, from scuba diving to hot air balloons. Whether it’s the concept of isothermal processes or the inverse relationship between pressure and volume, Boyle’s Law continues to amaze and inspire scientists and researchers around the world.
FAQs
1. What is Boyle’s Law?
Boyle’s Law states that the pressure of a gas is inversely proportional to its volume, provided the temperature remains constant. In simpler terms, as the volume of a gas increases, its pressure decreases, and vice versa.
2. Who discovered Boyle’s Law?
Boyle’s Law was named after Robert Boyle, an Irish scientist who first stated the principle in the 17th century. His experiments and observations laid the foundation for the understanding of gas behavior.
3. How does Boyle’s Law apply to scuba diving?
Boyle’s Law plays a crucial role in scuba diving. As a diver descends deeper into the water, the pressure increases. This causes the volume of air in their scuba tank to decrease. Adhering to Boyle’s Law helps divers prevent decompression sickness, commonly known as “the bends.”
4. Can Boyle’s Law be applied to other gases?
Yes, Boyle’s Law is applicable to all ideal gases as long as the temperature remains constant. It describes the behavior of gases under varying conditions and is a fundamental concept in the study of gas laws.
5. How is Boyle’s Law related to hot air balloons?
Boyle’s Law is vital for understanding how hot air balloons work. When the air inside the balloon is heated, it expands, causing the volume to increase. As a result, the pressure inside the balloon decreases, which allows it to rise in the air.
6. Is Boyle’s Law relevant in everyday life?
Absolutely! Boyle’s Law has real-world applications in various industries. It helps engineers design efficient ventilation systems, enables medical professionals to understand respiratory function, and assists in the manufacturing of aerosol cans, among other applications.
7. What happens if the temperature changes in Boyle’s Law?
Boyle’s Law assumes that the temperature remains constant, meaning if the temperature changes, the relationship between pressure and volume will no longer hold true. In such cases, the ideal gas law, which incorporates temperature, needs to be considered.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
