The study of gas laws is an essential branch of chemistry that deals with the behavior and properties of gases. Understanding gas laws is crucial not only for chemists but also for engineers, physicists, and even everyday individuals. Gas laws form the foundation of many important scientific principles and practical applications, ranging from understanding the behavior of weather systems to predicting the performance of engines and gas-filled containers.
In this article, we will explore 19 mind-blowing facts about gas laws that will not only expand your knowledge but also give you a glimpse into the fascinating world of gases. From the fundamental principles of Boyle’s Law and Charles’s Law to the intriguing concept of absolute zero, prepare to be amazed by the wonders of gas laws and their importance in various aspects of our lives.
Key Takeaways:
- Gases can expand to fill any space they’re in, and their behavior is governed by cool laws like Boyle’s Law and Charles’s Law. These laws help us understand how gases behave in different situations.
- Gas laws are super important in real life, from designing engines to scuba diving safety. Understanding them helps us solve problems and make things work better!
Gases expand to fill the available space.
One of the fundamental concepts of gas laws is that gases have the ability to expand and occupy the entire volume of the container in which they are confined. This property of gases is known as “expansion” and is a direct result of the random movements of gas particles.
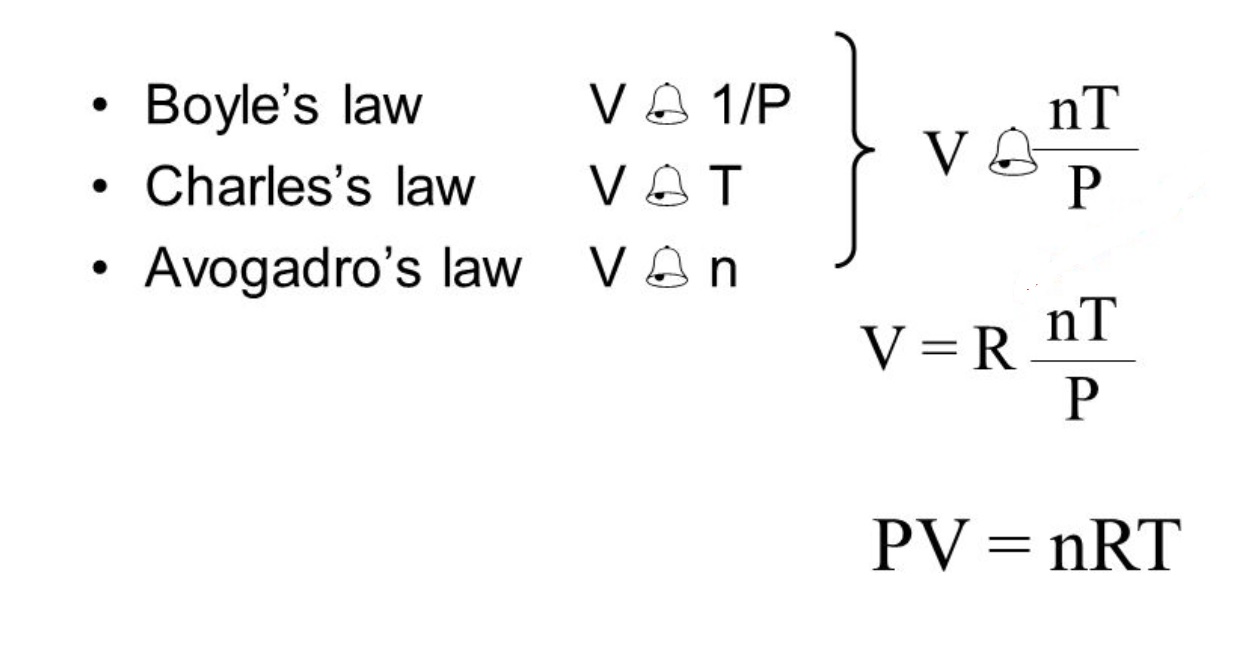
Boyle’s Law describes the relationship between pressure and volume.
According to Boyle’s Law, the pressure of a gas is inversely proportional to its volume, provided that the temperature and the amount of gas remain constant. This means that as the volume of a gas decreases, its pressure increases and vice versa.
Charles’s Law relates temperature and volume.
Charles’s Law states that the volume of a gas is directly proportional to its temperature, assuming that the pressure and the amount of gas are held constant. As the temperature of a gas increases, its volume expands, and as the temperature decreases, its volume decreases.
Gay-Lussac’s Law links pressure and temperature.
Gay-Lussac’s Law states that the pressure of a gas is directly proportional to its absolute temperature, while keeping the volume and the amount of gas constant. This means that as the temperature of a gas increases, its pressure also increases, and as the temperature decreases, its pressure decreases.
Avogadro’s Law states the relationship between volume and amount of gas.
According to Avogadro’s Law, at a constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of the gas. This means that as the number of moles of a gas increases, its volume increases as well.
The Ideal Gas Law combines all the gas laws.
The Ideal Gas Law, represented by the equation PV = nRT, brings together Boyle’s Law, Charles’s Law, and Gay-Lussac’s Law. It describes the behavior of an ideal gas by relating its pressure (P), volume (V), temperature (T), and number of moles (n).
Gases have measurable physical properties.
Gases have certain measurable properties such as pressure, volume, temperature, and density. These properties are crucial in understanding the behavior of gases and how they interact with each other and their surroundings.
Diffusion is the process of gas spreading out.
Diffusion is the spontaneous movement of gas particles from an area of higher concentration to an area of lower concentration. This process plays a fundamental role in various natural and industrial processes, including the mixing of gases.
Gases can be compressed.
Gases are highly compressible compared to liquids and solids. By applying external pressure, the volume of a gas can be reduced, leading to an increase in its density. This property of gases is extensively utilized in various applications, such as in gas cylinders.
The kinetic molecular theory explains gas behavior.
The kinetic molecular theory states that gases consist of small particles (atoms or molecules) in constant, random motion. It explains various properties of gases, including their expansion, diffusion, and pressure.
Gas pressure is caused by particle collisions.
The pressure exerted by a gas is a direct result of the numerous collisions between gas particles and the walls of the container. The more frequent and intense the collisions, the higher the pressure.
The concept of partial pressure is essential in gas mixtures.
When different gases are mixed together, each gas exerts a partial pressure proportional to its concentration. The total pressure exerted by the mixture is the sum of the individual partial pressures.
Gas laws are widely used in various scientific disciplines.
The gas laws have extensive applications in fields such as chemistry, physics, engineering, and environmental science. They are used to understand and predict the behavior of gases in different conditions.
The study of gas laws dates back to the 17th century.
The scientific study of gas laws began with the experiments and observations of scientists like Robert Boyle, Jacques Charles, and Joseph Louis Gay-Lussac in the 17th and 18th centuries. Their work laid the foundation for our understanding of gas behavior.
The Van der Waals equation accounts for gas deviations from ideal behavior.
The Van der Waals equation is an extension of the Ideal Gas Law that takes into account the non-ideal behavior of real gases due to intermolecular forces and molecular volume.
Absolute zero is the lowest possible temperature.
Absolute zero, at 0 Kelvin or -273.15 degrees Celsius, is the lowest temperature theoretically achievable. At this temperature, gas particles would cease all motion, rendering them unable to exert any pressure.
The speed of gas particles increases with higher temperatures.
According to the kinetic theory of gases, as the temperature of a gas increases, the average kinetic energy of its particles also increases. This leads to higher particle velocities and, consequently, a higher average speed.
Gas laws apply to both natural and man-made gases.
Whether it’s the gases found in the Earth’s atmosphere or those produced in industrial processes, the principles of gas laws apply universally to all types of gases. This allows scientists and engineers to make accurate predictions and calculations.
Understanding gas laws is vital for many practical applications.
From designing efficient combustion engines to controlling air pressure in scuba diving, a deep understanding of gas laws is essential for numerous practical applications. Knowledge of gas laws enables us to solve problems, optimize processes, and ensure safety in various industries.
Conclusion
In conclusion, gas laws play a fundamental role in our understanding of the behavior of gases. The concepts and principles behind these laws are not only applicable in chemistry labs but also have real-life implications in various fields like engineering, meteorology, and environmental science.Throughout this article, we have explored some mind-blowing facts about gas laws. We have learned about the three main gas laws: Boyle’s law, Charles’s law, and Gay-Lussac’s law, which describe the relationships between pressure, volume, and temperature. Additionally, we have delved into Avogadro’s law, the ideal gas law, and the significance of the gas constant.We have discovered intriguing facts such as the relationship between hot air balloons and gas laws, the role of gas laws in scuba diving, and the impact of gas laws on weather phenomena like storms and tornadoes. Furthermore, we have explored the behavior of gases at extremely low temperatures and high pressures, where quantum mechanics principles come into play.Studying gas laws opens up a fascinating world of how gases behave under different conditions and helps us make sense of the natural and man-made processes that involve gases. It is an exciting and essential area of study for anyone interested in understanding the properties and behavior of matter.
FAQs
Q: What are gas laws?
A: Gas laws are a set of mathematical relationships that describe the behavior of gases under different conditions, such as changes in pressure, volume, and temperature.
Q: What are the three main gas laws?
A: The three main gas laws are Boyle’s law, which relates pressure and volume; Charles’s law, which relates volume and temperature; and Gay-Lussac’s law, which relates pressure and temperature.
Q: How are gas laws applied in everyday life?
A: Gas laws have various applications in everyday life, such as in weather forecasting, scuba diving, and the design of equipment like gas tanks and air conditioners.
Q: What is the ideal gas law?
A: The ideal gas law combines Boyle’s, Charles’s, and Gay-Lussac’s laws into one equation and relates the pressure, volume, temperature, and number of gas particles in a sample.
Q: Are gas laws applicable to all types of gases?
A: Gas laws are most accurate for ideal gases that follow the ideal gas law. Real gases deviate from ideal behavior at high pressures or low temperatures, but modifications can be made to gas law equations to account for these deviations.
Q: How do gas laws relate to climate change?
A: Gas laws are involved in the study of the Earth’s atmosphere and its composition. Factors like the greenhouse effect, which relates to the properties of gases, including their ability to absorb and emit radiation, are important in understanding climate change.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


