Welcome to this fascinating exploration of Gay-Lussac’s Law! In the field of chemistry, certain laws form the backbone of our understanding of the behavior of gases. Gay-Lussac’s Law is one such fundamental principle that unveils the relationship between the pressure and temperature of a gas. Named after the French chemist Joseph Louis Gay-Lussac, this law provides invaluable insights into the behavior of gases at different temperatures.
In this article, we will delve into the depths of Gay-Lussac’s Law and uncover 15 mind-blowing facts that will leave you awe-inspired. From its origins and historical significance to its practical applications and impact on various industries, we will explore the wonders of this law. So, get ready to embark on a journey filled with interesting facts and intriguing discoveries!
Key Takeaways:
- Gay-Lussac’s Law explains how the pressure of a gas changes with temperature, helping us understand gas behavior in everyday life, from weather systems to hot air balloons.
- By following Gay-Lussac’s Law, scientists can predict how gases will react to temperature changes, making it a crucial concept in chemistry and providing insights into various practical applications.
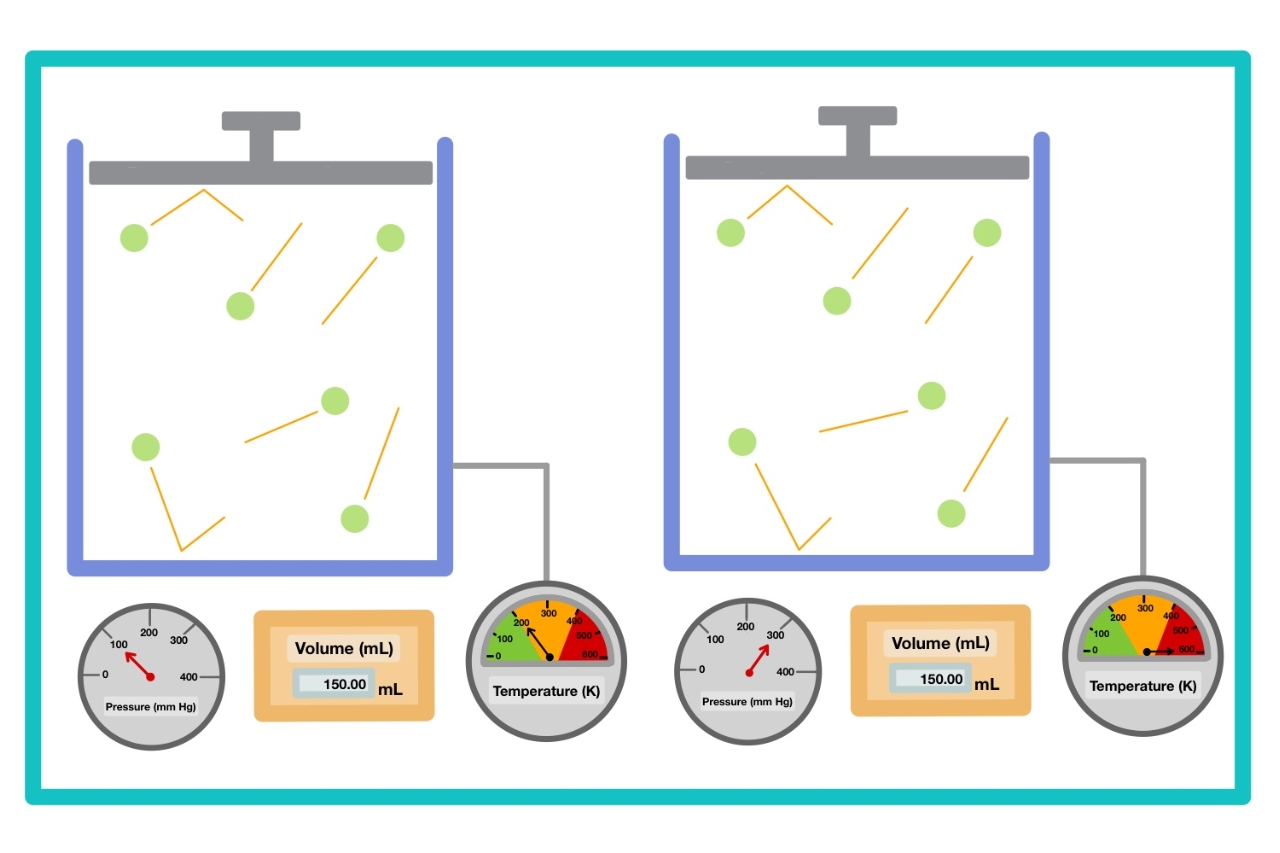
Gay-Lussac’s Law Explains the Relationship Between Temperature and Pressure
Gay-Lussac’s Law, also known as the pressure-temperature law, states that the pressure of a gas is directly proportional to its absolute temperature, when the volume and the amount of gas are constant. This law provides valuable insights into the behavior of gases under different temperature conditions.
It Was named after Joseph Louis Gay-Lussac
Gay-Lussac’s Law is named after the French chemist Joseph Louis Gay-Lussac, who first formulated this law in Gay-Lussac made significant contributions to the field of chemistry and is renowned for his work on gases.
The Law Can Be Expressed Using a Mathematical Equation
Gay-Lussac’s Law can be mathematically expressed as P1/T1 = P2/T2, where P1 and P2 represent the initial and final pressures, and T1 and T2 represent the initial and final temperatures, respectively. This equation helps in predicting the change in pressure with a change in temperature.
It Applies to Ideal Gases
Gay-Lussac’s Law is applicable to ideal gases, which are theoretical gases with no intermolecular forces and occupy negligible volume. Real gases deviate slightly from ideal behavior at high pressures and low temperatures.
It Can Be Used to Predict the Effect of Temperature on the Volume of a Gas
According to Gay-Lussac’s Law, when the volume of a gas is held constant, an increase in temperature will result in an increase in pressure. Conversely, a decrease in temperature will lead to a decrease in pressure.
It Describes the Behavior of Gases in a Closed System
Gay-Lussac’s Law applies to gases confined to a closed system, where the volume remains constant. It provides insights into how the pressure of a gas changes with temperature variations within such a system.
It Is Related to Charles’s Law
Gay-Lussac’s Law is closely related to another gas law known as Charles’s Law, which describes the relationship between the volume and temperature of a gas when pressure is held constant. Together, these laws form the basis of the ideal gas law.
It Helps Explain the Behavior of Gases in Various Applications
Gay-Lussac’s Law is significant in many practical applications. It helps in understanding the functioning of gas-powered engines, the behavior of gases in weather systems, the compression of gases in scuba diving, and the behavior of gases in various industrial processes.
It Was Developed Through Experimental Investigations
Gay-Lussac’s Law was formulated based on Gay-Lussac’s extensive experimental work. He conducted various experiments involving different gases and observed the direct relationship between pressure and temperature in a controlled environment.
It Can Be Used to Determine the Absolute Temperature of Gases
Gay-Lussac’s Law can be utilized to determine the absolute temperature of a gas by measuring its pressure at different known temperatures. This temperature scale, known as the absolute or Kelvin scale, is widely used in scientific calculations.
It Is Valid Only at Constant Volume
Gay-Lussac’s Law holds true only when the volume of the gas remains constant. If the volume changes, then the relationship between pressure and temperature becomes more complex and involves additional gas laws.
It Has Implications for the Ideal Gas Law
Gay-Lussac’s Law is an integral part of the ideal gas law, which combines the relationships between pressure, volume, and temperature for an ideal gas. The ideal gas law equation, PV = nRT, includes Gay-Lussac’s Law as one of its components.
It Is Relevant to the Study of Stoichiometry
Gay-Lussac’s Law is essential in stoichiometry, the area of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It helps in calculating the volumes of reactants and products based on their respective temperatures and pressures.
It Applies to Both Low and High Temperatures
Gay-Lussac’s Law is valid for gases at both low and high temperatures, as long as the volume remains constant. It allows scientists to understand the behavior of gases across a wide temperature range and predict how they will react under different conditions.
It Provides Insights Into the Behavior of Gases in Hot Air Balloons
Gay-Lussac’s Law plays a crucial role in understanding the behavior of gases used in hot air balloons. By heating the air inside the balloon, the temperature increases, causing the pressure to rise and allowing the balloon to ascend.
Conclusion
Gay-Lussac’s Law, named after the French chemist Joseph Louis Gay-Lussac, is a fundamental principle that explains the relationship between the pressure and temperature of a gas. This law states that the pressure of a gas is directly proportional to its temperature, provided that the volume and amount of gas remain constant. Understanding Gay-Lussac’s Law is essential in various fields of science and industry, including chemistry, physics, and engineering.
Through this article, we have explored 15 mind-blowing facts about Gay-Lussac’s Law. From its discovery and formulation to its real-life applications, Gay-Lussac’s Law serves as a cornerstone in our understanding of gas behavior. By delving into these fascinating facts, we can deepen our appreciation for the intricate and interconnected nature of the physical world.
So next time you come across a gas-related situation, remember the principles of Gay-Lussac’s Law and marvel at the wonders of the gas laws that govern our everyday lives.
FAQs
1. Who discovered Gay-Lussac’s Law?
Gay-Lussac’s Law was discovered and formulated by the French chemist Joseph Louis Gay-Lussac in the early 19th century.
2. What does Gay-Lussac’s Law state?
Gay-Lussac’s Law states that the pressure of a gas is directly proportional to its temperature, as long as the volume and amount of gas remain constant.
3. What are the real-life applications of Gay-Lussac’s Law?
Gay-Lussac’s Law has numerous applications in various fields. It is used to understand and predict the behavior of gases in industrial processes, such as chemical reactions and gas storage. It also helps in the design of pressure vessels and the study of weather patterns.
4. Can Gay-Lussac’s Law be applied to all gases?
Gay-Lussac’s Law is applicable to ideal gases, which follow certain assumptions. Real gases may deviate from the ideal behavior under extreme conditions.
5. How is Gay-Lussac’s Law related to other gas laws?
Gay-Lussac’s Law is one of the three fundamental gas laws, along with Boyle’s Law and Charles’s Law. These laws together form the basis of the ideal gas law equation.
Thirsty for more mind-blowing chemistry knowledge? Quench that curiosity by exploring Gay-Lussac's Law further! Unravel enigmatic facts about how this law applies to gases at constant volumes, perfect for those who love a good scientific mystery. Hungry for even more? Satisfy that appetite with an additional serving of 15 enigmatic facts about Gay-Lussac's Law of Gases. Each article promises a thrilling journey through the captivating world of chemistry, leaving readers inspired and amazed. Don't miss out on these incredible opportunities to expand your understanding of one of chemistry's most fundamental principles!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
