The Ideal Gas Law is a fundamental concept in the field of chemistry that describes the behavior of gases under different conditions. This law, also known as the general gas equation, relates the pressure, volume, temperature, and number of moles of an ideal gas. While it may seem like a dry and complex topic, delving into the details of the Ideal Gas Law can unravel some extraordinary facts that not only enhance our understanding of gases but also have practical applications in various fields. From the relationship between pressure and temperature to the concept of molar gas constants, this article will explore ten extraordinary facts about the Ideal Gas Law that will leave you amazed at the wonders of the gaseous world.
Key Takeaways:
- The Ideal Gas Law is a super important equation that helps scientists understand and predict how gases behave under different conditions. It’s like a special math code for gases!
- By using the Ideal Gas Law, scientists and engineers can solve tricky gas problems and design cool new technologies. It’s like having a secret formula for understanding and working with gases!
The Ideal Gas Law is a fundamental equation in thermodynamics.
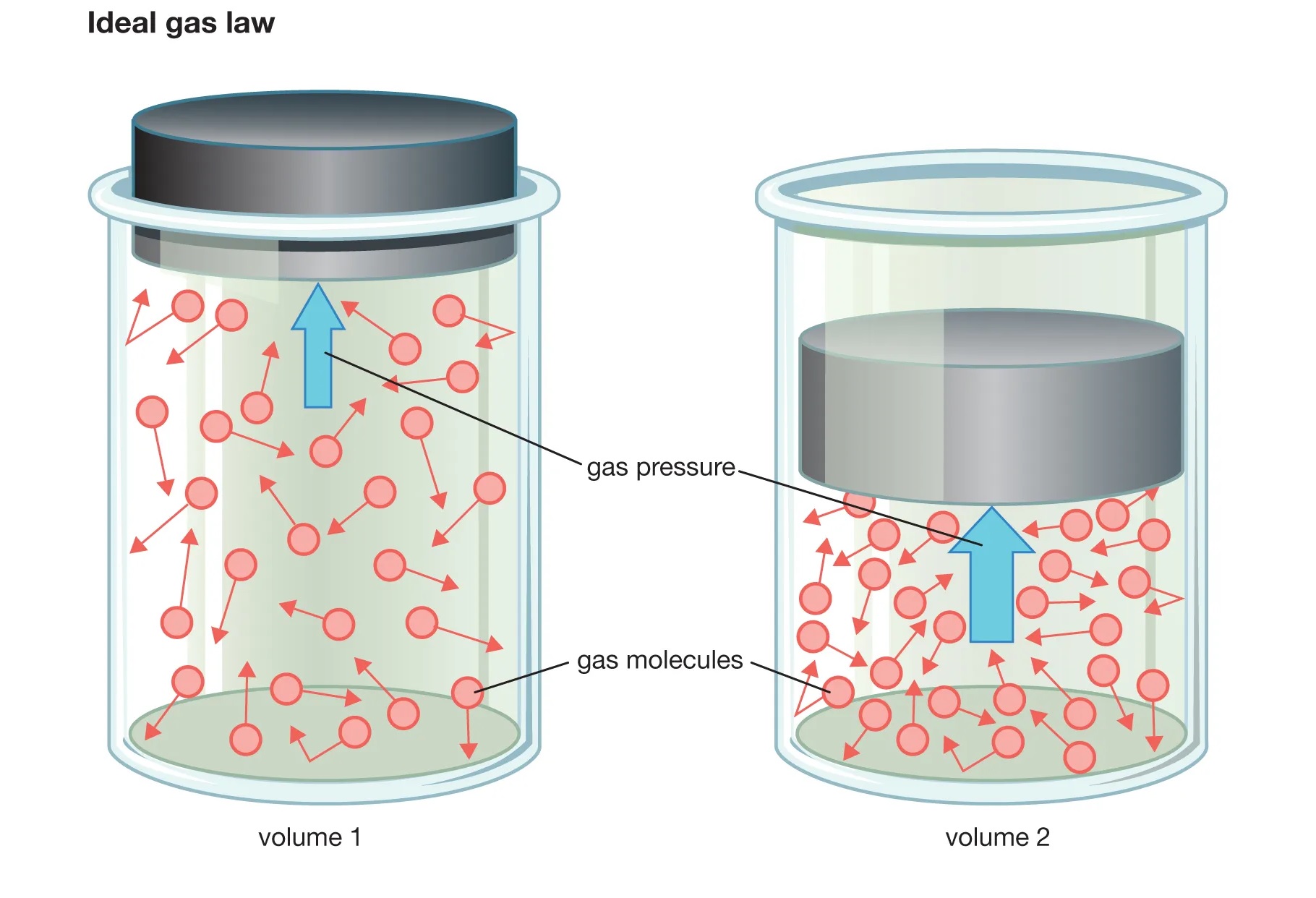
The Ideal Gas Law, represented by the equation PV = nRT, relates the pressure (P), volume (V), temperature (T), and number of moles (n) of an ideal gas. It provides a mathematical relationship between these variables, allowing scientists and researchers to analyze and predict the behavior of gases under various conditions.
The Ideal Gas Law assumes certain ideal conditions.
The Ideal Gas Law assumes that the gas particles are point masses with negligible volume, that they do not interact with each other, and that the interactions with their container are perfectly elastic. While these assumptions may not hold true for all gases in real-life scenarios, the Ideal Gas Law serves as a useful approximation in numerous practical applications.
The Ideal Gas Law can be derived from basic principles.
The Ideal Gas Law can be derived from the principles of kinetic theory, which describes the behavior of gas particles. By considering the average kinetic energy of the gas particles and the relationship between pressure and force, one can arrive at the mathematical expression of the Ideal Gas Law.
The Ideal Gas Law allows for the calculation of unknown variables.
By rearranging the equation PV = nRT, scientists and engineers can solve for any of the four variables (pressure, volume, temperature, or number of moles) if the other three are known. This enables them to make predictions, perform calculations, and design systems involving gases.
The Ideal Gas Law holds true for a wide range of gases.
While the assumptions of the Ideal Gas Law may not perfectly match the behavior of all gases, it is remarkably accurate for many common gases, including nitrogen, oxygen, hydrogen, and helium. This makes it a valuable tool in fields such as chemistry, physics, and engineering.
The Ideal Gas Law is applicable under varying conditions.
The Ideal Gas Law can be used to analyze the behavior of gases at different temperatures, pressures, and volumes. It provides insights into how gases behave under changing conditions and aids in the design and optimization of various industrial processes.
The Ideal Gas Law helps in understanding gas mixtures.
By applying the Ideal Gas Law to gas mixtures, researchers can determine the composition and behavior of different gases within a mixture. This is crucial in fields such as atmospheric science, gas chromatography, and chemical engineering.
The Ideal Gas Law is essential in the study of thermodynamics.
The Ideal Gas Law serves as a foundation for many thermodynamic principles and equations. It is extensively used in the analysis of heat engines, refrigeration systems, ideal gas processes, and other thermodynamic systems.
The Ideal Gas Law has practical applications in various industries.
The Ideal Gas Law finds applications in fields such as aerospace engineering, environmental science, pharmaceuticals, chemical manufacturing, and many more. It helps researchers understand the behavior of gases and design efficient processes and systems.
The Ideal Gas Law is a powerful tool for scientific research and problem-solving.
The Ideal Gas Law provides a framework for understanding and predicting the behavior of gases. It enables scientists and engineers to tackle complex problems, develop new technologies, and advance our understanding of the physical world.
Conclusion
In conclusion, the Ideal Gas Law is a fundamental concept in chemistry that helps us understand and predict the behavior of gases. With its equation PV = nRT, it links pressure, volume, temperature, and the number of moles of gas. The law provides crucial insights into gas properties and allows scientists and engineers to design and optimize various processes involving gases.The 10 extraordinary facts about the Ideal Gas Law presented in this article shed light on its significance and applications. From the laws’ universality to its role in determining the behavior of mixtures and reactions, the Ideal Gas Law is an essential tool in the field of chemistry. Understanding these facts not only deepens our knowledge of gases but also enhances our ability to manipulate and control them for industrial, environmental, and scientific purposes.So, whether you are a student studying chemistry or an industry professional working with gases, familiarizing yourself with the Ideal Gas Law will undoubtedly broaden your horizons and enable you to make informed decisions in various gas-related situations.
FAQs
Q: What is the Ideal Gas Law?
A: The Ideal Gas Law is an equation that relates the pressure, volume, temperature, and number of moles of gas in a system. It is represented by the equation PV = nRT.
Q: What does the Ideal Gas Law explain?
A: The Ideal Gas Law explains the relationships between the properties of gases. It helps predict how changes in pressure, volume, temperature, and the amount of gas affect each other.
Q: Is the Ideal Gas Law applicable to all gases?
A: The Ideal Gas Law is an approximation that works well for most gases under normal conditions. However, it becomes less accurate at high pressures or low temperatures.
Q: How is the Ideal Gas Law useful in real life?
A: The Ideal Gas Law is vital in various practical applications. It is used in industries such as manufacturing, pharmaceuticals, and environmental engineering to optimize gas-related processes and ensure their efficiency.
Q: Can the Ideal Gas Law be applied to mixtures of gases?
A: Yes, the Ideal Gas Law can be applied to mixtures of gases. In such cases, the total pressure and volume are considered as the sum of the individual pressures and volumes of the gases present.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
