Chemistry enthusiasts often find themselves captivated by the intricate world of chemical bonds. One fundamental concept that continues to intrigue scientists and students alike is bond order. Bond order, a term used to describe the strength and stability of a chemical bond, plays a vital role in understanding the properties and behavior of a molecule.
In this article, we will explore 12 extraordinary facts about bond order that will enhance your understanding of this key concept. From its significance in determining the type of bond to its relationship with molecular geometry, we will delve into fascinating aspects of bond order that will leave you fascinated by the wonders of chemistry. So, if you’re ready to dive into the world of chemical bonding, let’s begin our exploration of the extraordinary facts about bond order!
Key Takeaways:
- Bond order determines how strong and stable a chemical bond is, influencing a molecule’s energy and properties. It’s like the secret code that tells us how tightly atoms are holding hands!
- Bond order affects everything from bond length to reactivity and even magnetism. It’s like the magic ingredient that shapes the behavior and properties of molecules, making chemistry super fascinating!
Bond Order Determines Bond Strength and Stability
Bond order is a measure of the strength and stability of a chemical bond between two atoms. It helps determine the overall energy of a molecule and influences its physical and chemical properties.
Bond Order Can Be Calculated
Bond order can be calculated using various methods, such as the Lewis structure, molecular orbital theory, or the Valence Bond theory. These calculations provide insights into the nature of chemical bonding.
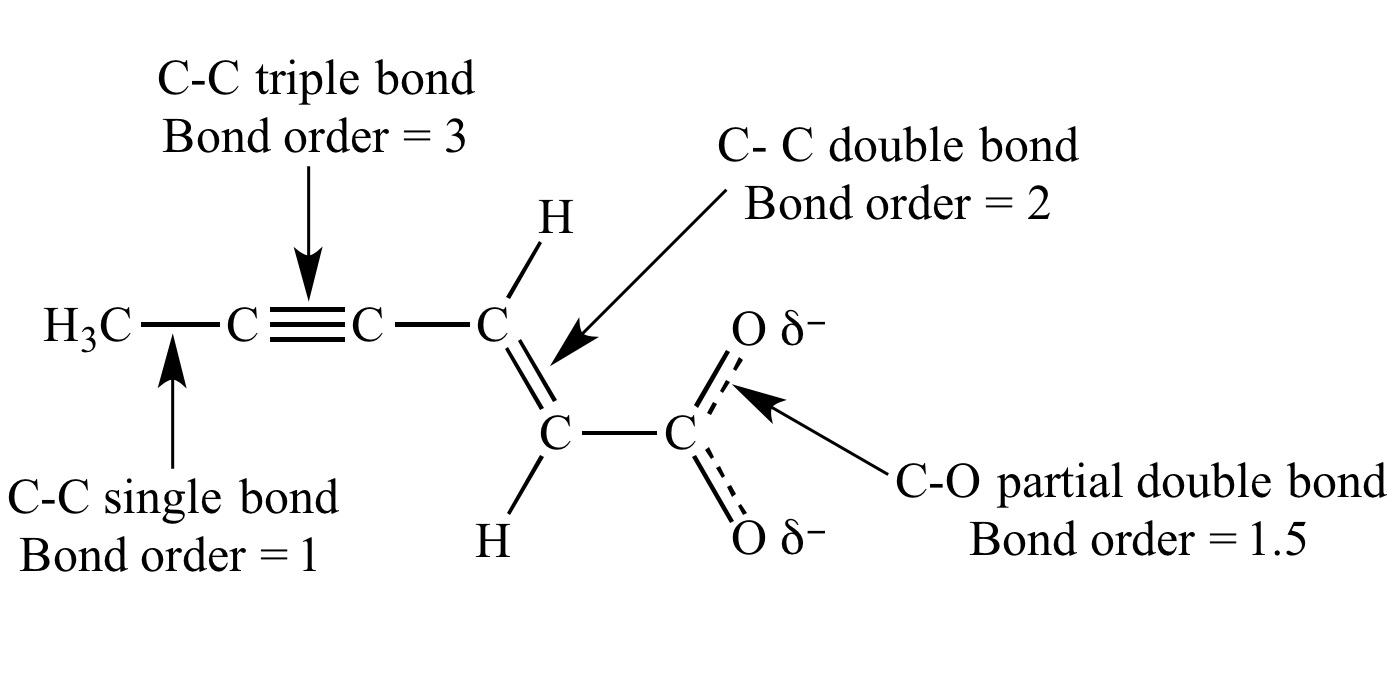
Bond Order Defines the Type of Bond
Bond order helps classify the type of bond between atoms. A bond order of 1 indicates a single bond, whereas a bond order of 2 represents a double bond, and a bond order of 3 indicates a triple bond.
Bond Order Determines Bond Length
The bond length between two atoms is inversely proportional to the bond order. Higher bond order results in shorter bond length, indicating stronger attraction and tighter bonding.
Bond Order Helps Predict Bond Energy
The higher the bond order, the greater the bond energy. Bond energy is the amount of energy required to break a bond between two atoms. It is influenced by the strength of the bond, which is determined by the bond order.
Bond Order Influences Molecular Geometry
The bond order affects the shape and geometry of molecules. Different bond orders lead to variations in bond angles and molecular structures, influencing the overall shape and properties of the molecule.
Bond Order Plays a Role in Chemical Reactivity
The bond order influences the reactivity of molecules. Higher bond order implies stronger bonds and less reactivity, whereas lower bond order indicates weaker bonds and higher reactivity.
Bond Order Determines Magnetism
Some molecules exhibit magnetic properties due to their bond order. Paramagnetic molecules have unpaired electrons and are attracted to a magnetic field, whereas diamagnetic molecules have paired electrons and are not affected by a magnetic field.
Bond Order Can Change in Chemical Reactions
Bond order can change during chemical reactions as bonds are formed or broken between atoms. This alteration affects the overall energy and stability of the reactants and products.
Bond Order Helps Explain Bond Strength Discrepancies
Understanding bond order can explain discrepancies in bond strength observed for similar bonds in different molecules. Differences in bond order account for variations in bond strength and reactivity.
Bond Order Can Influence Polarity
Bond order affects the polarity of a molecule. Greater bond order leads to a more polar molecule with uneven electron distribution, resulting in stronger intermolecular forces.
Bond Order Impacts Physical and Chemical Properties
The bond order directly influences properties such as melting point, boiling point, solubility, and conductivity. Higher bond order molecules typically have higher melting and boiling points, lower solubility, and higher conductivity.
Conclusion
In conclusion, bond order is a crucial concept in chemistry that helps us understand the strength and stability of chemical bonds. It provides valuable insights into the nature of molecules and their properties. By determining the number of shared or unshared electrons between atoms, bond order allows us to predict the type of bond present, whether it is single, double, or triple.Through this article, we have explored 12 extraordinary facts about bond order. We have learned how bond order influences bond length, bond energy, and molecular stability. Additionally, we have seen how bond order can vary in different molecules and how it affects the reactivity and physical properties of substances.Understanding the intricacies of bond order opens up a world of possibilities in various fields, such as drug development, materials science, and environmental research. It is through this knowledge that we can continue to push the boundaries of scientific discovery and innovation.
FAQs
Q: What is bond order?
A: Bond order is a measure of the number of chemical bonds between a pair of atoms in a molecule.
Q: How is bond order calculated?
A: Bond order is calculated by subtracting the number of electrons in antibonding orbitals from the number of electrons in bonding orbitals and dividing the result by two.
Q: What does a higher bond order indicate?
A: A higher bond order indicates a stronger and shorter bond between atoms, indicating greater stability and higher bond energy.
Q: How does bond order affect bond length?
A: Bond order and bond length are inversely proportional. As bond order increases, bond length decreases.
Q: Can bond order be fractional?
A: No, bond order is always a whole number. It can be 0 for non-bonding electrons, 1 for a single bond, 2 for a double bond, or 3 for a triple bond.
Q: Does bond order affect molecular reactivity?
A: Yes, higher bond orders generally indicate increased molecular stability and lower reactivity. Multiple bonds can also facilitate reactions involving breaking and forming of chemical bonds.
Q: Are bond orders consistent within a molecule?
A: Bond orders can vary within a molecule depending on the type of bond and the atoms involved. Different bonds within a molecule can have different bond orders.
Q: Can bond order explain the physical properties of substances?
A: Yes, bond order influences the physical properties of substances such as boiling point, melting point, and solubility. Higher bond order generally leads to increased intermolecular forces and higher melting and boiling points.
Q: Does bond order play a role in drug development?
A: Yes, bond order affects the interaction of drugs with target molecules in the body, influencing their effectiveness and potential side effects.
Q: Can bond order change during chemical reactions?
A: Yes, bond order can change during chemical reactions as atoms rearrange and form new bonds.
Unraveling bond order's mysteries is just the beginning! Dive deeper into chemistry's fascinating world by exploring electron configurations, a fundamental concept that determines an atom's chemical behavior. Discover how bond length affects molecular properties and shapes, influencing everything from boiling points to reactivity. Finally, master Lewis structures, powerful visual representations that help predict molecular geometry and bonding patterns. Ready to continue your chemistry journey?
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
