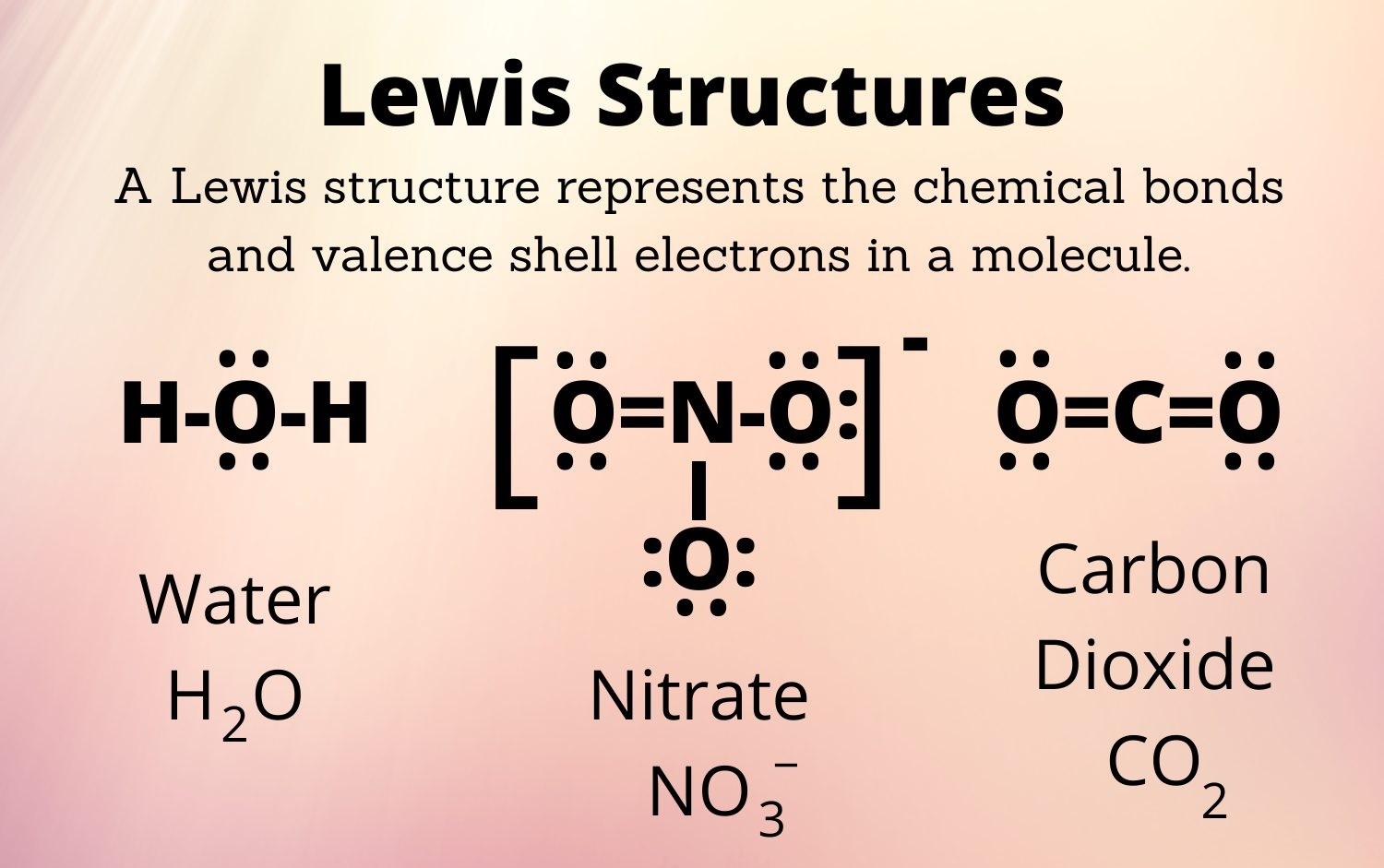
When studying chemistry, one of the fundamental concepts that students encounter is Lewis structures. These diagrams, also known as Lewis dot structures or electron dot structures, provide a visual representation of how atoms bond to form molecules. They help us understand how electrons are shared or transferred between atoms, giving insights into the structure and properties of compounds.
In this article, we are going to delve into the world of Lewis structures and explore 12 intriguing facts that will enhance your understanding of this essential tool in chemistry. We will unravel the history behind their development, examine the rules for drawing Lewis structures, dive into their role in determining molecular geometry, and uncover some lesser-known aspects about these fascinating diagrams. So let’s embark on this educational journey and unravel the secrets of Lewis structures!
Key Takeaways:
- Lewis structures show how atoms share electrons to form molecules, helping us understand their shapes and properties. They’re like electron blueprints for molecules!
- By using Lewis structures, scientists can predict how molecules behave in reactions and whether they are polar or nonpolar. It’s like a secret code for understanding chemical secrets!
Fascinating The Concept of Lewis Structures
The concept of Lewis structures, also known as electron dot structures or Lewis dot structures, was introduced by the American chemist Gilbert N. Lewis in This revolutionary approach provides a simplified representation of the valence electrons in an atom.
Key Components of Lewis Structures
Lewis structures consist of various elements, including symbols to represent atoms, dots to represent valence electrons, and lines to indicate chemical bonds between atoms. These components allow chemists to visually depict the arrangement of electrons in a molecule.
Surprising Lewis Structures and Octet Rule
The octet rule is a fundamental principle in Lewis structures. It states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons, similar to the noble gases. Lewis structures help us understand how atoms achieve this stable state through the sharing or transfer of electrons.
Representing Single Bonds
In Lewis structures, single covalent bonds are represented by a single line (-) between two atoms. This signifies the sharing of two electrons, one from each of the bonded atoms.
Interesting Multiple Bond Representation
When atoms share more than one pair of electrons, multiple bonds are formed. In Lewis structures, double bonds are represented by two lines (=), while triple bonds are depicted by three lines (?).
Exception to the Octet Rule
While most atoms strive for an octet of valence electrons, there are exceptions. Some atoms, such as hydrogen (H) and helium (He), only require two electrons for stability. Additionally, elements beyond the second period (such as phosphorus and sulfur) can exceed the octet rule and accommodate more than eight electrons around their central atom.
Intriguing Lewis Structures and Molecular Geometry
Lewis structures help us predict the molecular geometry of a compound. By understanding the arrangement of atoms and lone pairs, we can determine whether a molecule is linear, trigonal planar, tetrahedral, or exhibits other spatial arrangements.
Formal Charge Calculations
Lewis structures play a vital role in calculating formal charges. Formal charge is a mathematical concept that helps us determine which resonance structure is the most stable. By assigning formal charges to atoms in a molecule, chemists can assess the distribution of electrons.
Surprising Lewis Structures and Chemical Bonding
Lewis structures provide valuable insights into the nature of chemical bonding. They help us understand the sharing or transfer of electrons between atoms, which in turn influences the strength and stability of chemical bonds.
Relationship to VSEPR Theory
Lewis structures are closely related to the VSEPR (Valence Shell Electron Pair Repulsion) theory. Both theories contribute to our understanding of molecular shapes and the arrangement of electron pairs around the central atom.
Interesting Predicting Polarity with Lewis Structures
Lewis structures can be used to predict the polarity of a molecule. By examining the distribution of electrons and the electronegativity difference between atoms, we can determine whether a molecule is polar or nonpolar.
Lewis Structures and Chemical Reactions
Lewis structures play a crucial role in understanding chemical reactions. They help us identify the breaking and formation of chemical bonds, as well as the movement of electrons during a reaction.
Conclusion
In conclusion, understanding Lewis structures is crucial for anyone studying chemistry. These diagrams provide a visual representation of how atoms connect and arrange themselves in molecules. By following a set of rules, we can accurately depict the bonding and electron distribution within a molecule.Through this article, we have explored 12 intriguing facts about Lewis structures. We have learned how to determine the central atom, assign lone pairs and bonding pairs, and represent multiple bonds. We have also discussed resonance structures, formal charges, and the octet rule.By mastering Lewis structures, chemists can predict the geometry, polarity, and reactivity of molecules. This knowledge is essential for a wide range of applications, including drug discovery, environmental analysis, and material science.So, whether you’re a student aiming to excel in chemistry or simply fascinated by the inner workings of molecules, delving into Lewis structures will open up a whole new world of understanding and exploration.
FAQs
Q: What is a Lewis structure?
A: A Lewis structure is a diagram that represents the bonding and electron distribution within a molecule. It uses lines to represent bonds between atoms and dots to symbolize lone pairs of electrons.
Q: How do you determine the central atom in a Lewis structure?
A: The central atom in a Lewis structure is usually the one with the lowest electronegativity or the atom that can make the most bonds. However, there are exceptions, so it’s important to consider specific guidelines for different molecules.
Q: Can an atom have more than an octet of electrons in a Lewis structure?
A: Yes, atoms in the third period and beyond of the periodic table can have an expanded octet and accommodate more than eight electrons. This is because they have d orbitals available for bonding.
Q: What is the purpose of formal charges in Lewis structures?
A: Formal charges help us determine the most stable arrangement of electrons in a molecule. They enable us to identify potential sites of electron transfer and provide insights into its reactivity.
Q: Are resonance structures real representations of a molecule?
A: Resonance structures are not individual molecules but different ways to depict the same molecule. They show the delocalization of electrons and the stability of the molecule.
Q: Can you have a Lewis structure without any lone pairs?
A: Yes, some molecules do not have any lone pairs. These molecules typically consist of two atoms bonded together, such as diatomic molecules like oxygen (O?) or nitrogen (N?).
Mastering Lewis structures is just the beginning of your chemistry journey. Unbelievable facts about Lewis structures await, ready to challenge what you thought you knew. Calculating formal charges becomes captivating when you explore its intricacies. Resonance structures hold intriguing secrets that will reshape your understanding of molecular bonding.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
