When it comes to understanding the intricacies of chemistry, the study of secondary structure holds a significant place. Secondary structure refers to the folding patterns of proteins, DNA, and RNA, which play vital roles in their functionality. Exploring the realm of secondary structure can unlock a world of fascinating facts that shed light on the molecular universe.
This article aims to take you on a journey through 18 captivating facts about secondary structure. From the discovery of alpha helices and beta sheets to the influence of secondary structure on protein stability and function, we will unravel the hidden mysteries behind these essential components of biomolecules.
So, buckle up and prepare to delve into the captivating world of secondary structure as we explore the forces that shape these molecular structures and their impact on the complex web of life.
Key Takeaways:
- Proteins have cool shapes! The Alpha Helix and Beta Sheet are like the superheroes of protein folding, determining their stability and function. It’s like a protein’s secret identity!
- Proteins are picky about their shapes. Factors like amino acid sequence and environmental conditions influence how proteins fold. It’s like a puzzle where every piece has its own special spot!
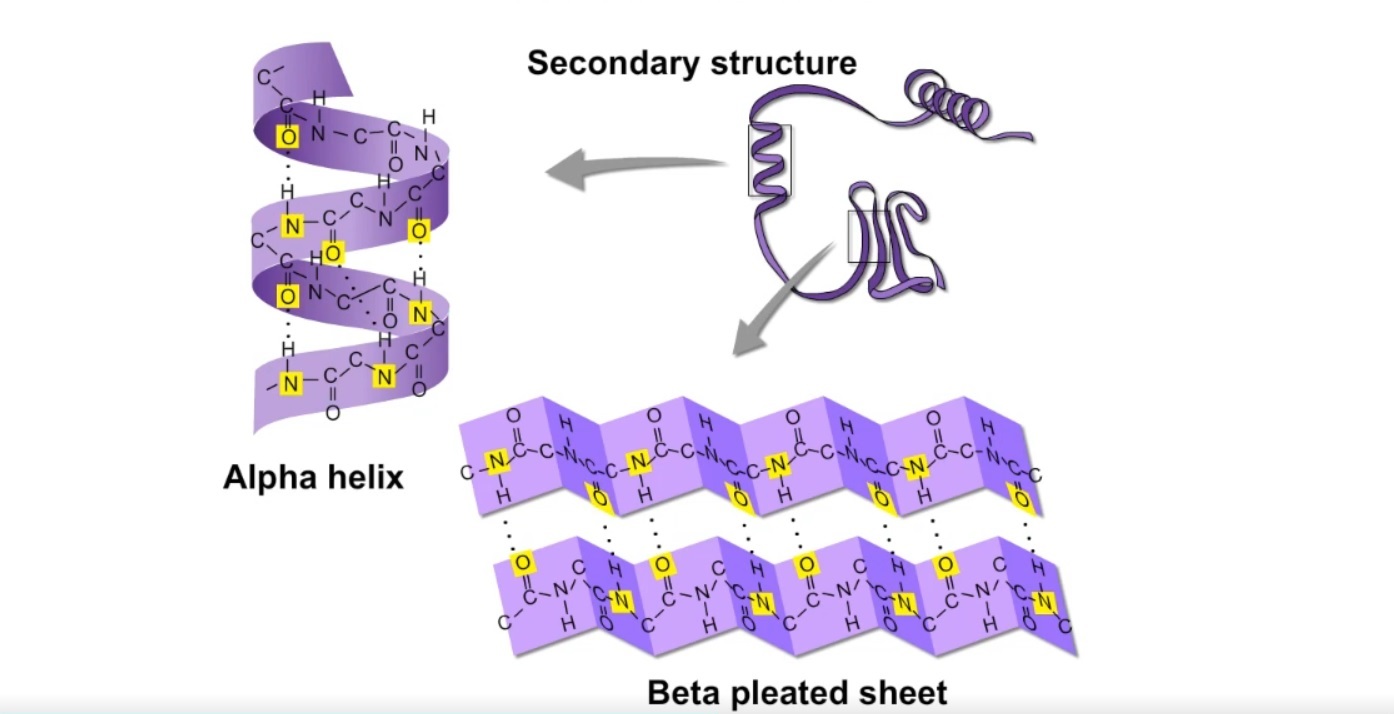
The Alpha Helix and Beta Sheet are the two main types of secondary structures in proteins.
Secondary structure refers to the local folding patterns of amino acid chains in a protein. The Alpha Helix is a right-handed spiral shape, while the Beta Sheet consists of multiple strands running alongside each other.
Secondary structure plays a crucial role in determining a protein’s overall shape and function.
The specific arrangement of the Alpha Helix and Beta Sheet regions contributes to the protein’s stability and its ability to interact with other molecules.
The Alpha Helix was first proposed by Linus Pauling and Robert Corey in 1951.
These two scientists discovered the regular repeating pattern of hydrogen bonding that gives rise to the coiled structure of the Alpha Helix.
Beta Sheets can be either parallel or antiparallel.
In a parallel Beta Sheet, adjacent strands run in the same direction, while in an antiparallel Beta Sheet, adjacent strands run in opposite directions, creating a more stable structure.
The Ramachandran Plot is a graphical representation of the allowed conformations of amino acids in a protein’s secondary structure.
It helps predict the possible angles of rotation for phi (?) and psi (?), which determine the overall folding pattern of the protein.
Beta Turns are structural motifs that connect strands of a Beta Sheet.
They involve a change in direction of the polypeptide chain, allowing for the formation of a compact structure.
Protein domains often have distinct secondary structures.
A domain is a structurally and functionally independent unit within a protein, and secondary structures play a significant role in defining their boundaries.
Secondary structures can exhibit local motifs, such as the helix-turn-helix and the Greek key.
These motifs occur when specific arrangements of secondary structure elements come together to form recognizable patterns.
Secondary structures are stabilized by hydrogen bonding between amino acid residues.
This bonding occurs between the carbonyl oxygen of one residue and the amide hydrogen of another residue, creating a stable interaction.
The formation of secondary structures is influenced by various factors, including amino acid sequence and environmental conditions.
Different amino acids have varying propensities for adopting specific secondary structure elements, and factors like temperature and pH can also impact their formation.
The Protein Data Bank (PDB) is a valuable resource for studying protein structures and secondary structure elements.
It contains a vast collection of experimentally determined three-dimensional structures of proteins from diverse organisms.
Certain diseases and mutations can disrupt secondary structure and lead to protein misfolding.
These misfolded proteins can aggregate and interfere with cellular functions, contributing to conditions like Alzheimer’s, Parkinson’s, and prion diseases.
The Circular Dichroism (CD) technique is commonly used to study the presence and stability of secondary structures in proteins.
CD measures the differential absorption of left and right circularly polarized light, providing information about the protein’s structural properties.
The secondary structure of a protein can sometimes be predicted computationally using algorithms based on statistical analysis and machine learning.
These methods analyze protein sequences and compare them to known structures to infer the most likely secondary structure elements.
Secondary structures can undergo conformational changes in response to ligand binding or enzymatic reactions.
These changes are essential for the proper functioning of proteins and can affect their interactions with other molecules.
The formation of secondary structures is a dynamic process influenced by thermodynamics and kinetics.
Factors such as temperature, pressure, and the presence of other molecules can affect their stability and folding rates.
Specific amino acid residues, called helix-breakers or helix-promoters, can impact the formation of secondary structures.
These residues have unique properties that either favor or hinder the formation of helices or sheets in the protein structure.
Secondary structures can vary in length, with Alpha Helices typically being 10 to 45 amino acids long and Beta Sheets ranging from 4 to 22 amino acids.
The length and arrangement of secondary structure elements contribute to the overall complexity and functionality of the protein.
These 18 fascinating facts showcase the importance and intricacies of secondary structure in protein biology. Understanding how proteins fold and adopt specific shapes is essential for unraveling their biological functions and developing therapeutic interventions. Explore the world of secondary structure and delve into the fascinating complexities that underlie the functioning of proteins.
Conclusion
In conclusion, secondary structure is a fundamental concept in chemistry that plays a crucial role in understanding the properties and behavior of biomolecules. It refers to the local folding patterns within a protein or nucleic acid molecule, specifically the arrangements of its backbone atoms. Two common types of secondary structures are ?-helices and ?-sheets, which are stabilized by hydrogen bonding.Secondary structure is essential for the proper functioning of biomolecules. It determines their stability, flexibility, and ability to interact with other molecules. Understanding the principles and characteristics of secondary structures is key to unraveling the complex mechanisms of biological processes and developing new drugs and therapies.By studying secondary structure, scientists have made significant advancements in fields such as biochemistry, molecular biology, and medicinal chemistry. The discovery and analysis of secondary structures have paved the way for groundbreaking research and remarkable discoveries in the realm of life sciences.In summary, secondary structure is an intriguing and vital aspect of chemistry that continues to captivate scientists and drive innovation in various scientific disciplines. Its intricate details and fascinating properties have unlocked numerous doors to unravel the mysteries of life itself.
FAQs
1. What is the importance of secondary structure in biology?
Secondary structure is crucial in biology as it determines the stability, flexibility, and functionality of biomolecules. It plays a key role in protein folding, DNA replication, RNA structure, and enzyme catalysis.
2. How are ?-helices and ?-sheets formed?
?-helices and ?-sheets are formed through hydrogen bonding between different regions of a protein or nucleic acid molecule. In ?-helices, hydrogen bonds form between the carbonyl oxygen and amide hydrogen atoms in the backbone, creating a helical structure. In ?-sheets, hydrogen bonds form between adjacent strands, resulting in a pleated sheet-like structure.
3. Can secondary structure prediction be accurate?
Secondary structure prediction methods have greatly improved in recent years, and they can provide accurate predictions for many proteins. However, the accuracy of predictions may vary depending on the complexity and size of the protein, as well as the availability of sequence information.
4. How does secondary structure influence protein function?
The specific arrangement of secondary structures within a protein is critical for its function. It determines how the protein interacts with other molecules, including substrates, enzymes, and receptors. It also affects the protein’s stability, conformational changes, and binding affinity.
5. Are there any other types of secondary structures?
While ?-helices and ?-sheets are the most common secondary structures, there are other less prevalent structures such as 310 helices, polyproline helices, and turns. These structures exhibit unique geometries and play specific roles in protein functionality.
Unraveling secondary structure's secrets is just the beginning of your journey into the captivating world of proteins. Dive deeper into protein folding mysteries, hydrogen bonding intricacies, and peptide bonds' crucial role in shaping life's building blocks. Each topic offers a unique perspective on the complex dance of amino acids, inviting you to explore further and expand your understanding of these fundamental concepts. So, whether you're a curious student, a seasoned researcher, or simply a science enthusiast, prepare to be amazed by the hidden wonders waiting to be discovered in the realm of proteins.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
