Hydrogen bonding is a crucial phenomenon in chemistry that plays a fundamental role in various biological, physical, and chemical processes. It occurs when a hydrogen atom covalently bonded to an electronegative atom (such as nitrogen, oxygen, or fluorine) interacts with another electronegative atom nearby. While hydrogen bonding may seem like a simple concept, it is a fascinating topic with many intriguing aspects.
In this article, we will delve into 12 fascinating facts about hydrogen bonding that will deepen your understanding of this essential concept. From its crucial role in stabilizing DNA structure to its influence on the properties of water, hydrogen bonding has far-reaching implications across different disciplines. So, let’s explore these intriguing facts and gain a deeper appreciation for the wonders of hydrogen bonding.
Key Takeaways:
- Hydrogen bonding is a strong force responsible for water’s unique properties, DNA structure, and protein functionality. It affects physical properties and plays a crucial role in drug design and biological systems.
- Despite its misleading name, hydrogen bonding is not a true chemical bond. It influences the structure of ice, affects chemical reactions, and can be found in various substances beyond water and biological molecules.
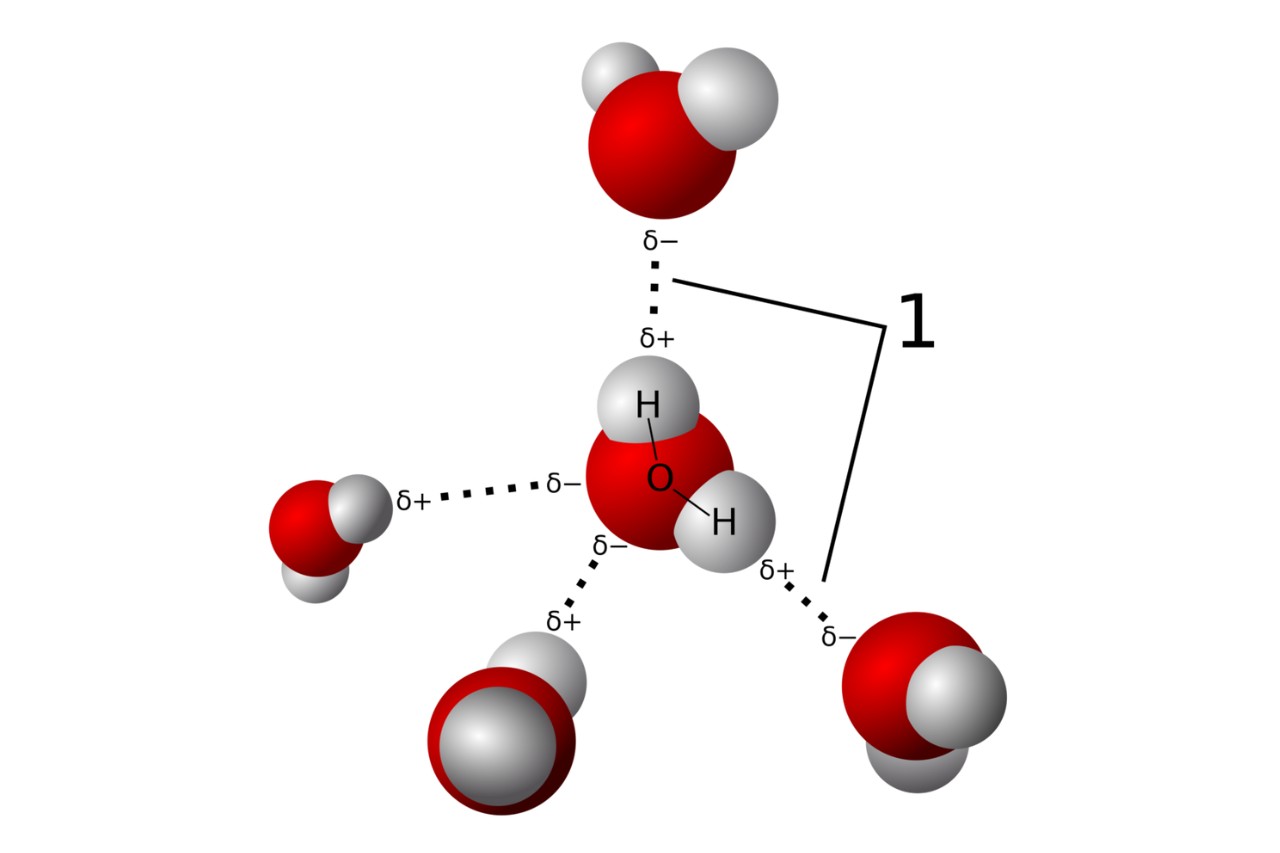
Hydrogen Bonding is not a true bond
Contrary to its name, hydrogen bonding is not an actual chemical bond. It is more accurately described as an attractive force between a hydrogen atom and an electronegative atom such as oxygen, nitrogen, or fluorine.
Hydrogen Bonding is stronger than other intermolecular forces
Hydrogen bonds are significantly stronger than other intermolecular forces such as dipole-dipole interactions and London dispersion forces. This is due to the large electronegativity difference between hydrogen and the electronegative atom it is bonded to.
Hydrogen Bonding is responsible for water’s unique properties
The hydrogen bonding in water gives it its high boiling point, surface tension, and ability to dissolve many substances. These properties are crucial for supporting life as we know it.
Hydrogen Bonding determines the structure of DNA
The hydrogen bonds between complementary base pairs (adenine-thymine and guanine-cytosine) play a crucial role in the double helix structure of DNA. They provide stability and allow for the replication and transcription of genetic information.
Hydrogen Bonding affects the properties of proteins
Hydrogen bonds play a vital role in protein folding and determining its three-dimensional structure. They contribute to the stability and functionality of proteins.
Hydrogen Bonding is important in chemical reactions
Hydrogen bonding can influence the rate and outcome of chemical reactions. It can affect the activation energy required for a reaction and facilitate the formation of reaction intermediates.
Hydrogen Bonding occurs in biological systems
Hydrogen bonding is prevalent in biological systems. It is involved in protein-ligand interactions, enzyme-substrate binding, and the specificity of molecular recognition.
Hydrogen Bonding is found in many substances
Hydrogen bonding is not limited to water and biological molecules. It can occur in many other substances, such as alcohols, carboxylic acids, and amides.
Hydrogen Bonding can affect physical properties
The presence of hydrogen bonding can significantly influence the physical properties of a substance, including its boiling point, melting point, viscosity, and solubility.
Hydrogen Bonding is essential in drug design
Hydrogen bonding plays a crucial role in drug-target interactions. Designing molecules that can form specific hydrogen bonds with target proteins is a key strategy in drug discovery.
Hydrogen Bonding is responsible for the unique properties of ice
Hydrogen bonding causes water molecules to arrange themselves in a crystalline lattice when freezing, leading to the expansion and lower density of ice compared to liquid water.
Hydrogen Bonding is influenced by temperature and pressure
The strength and prevalence of hydrogen bonding can be affected by temperature and pressure. Changes in these conditions can alter the extent of hydrogen bonding interactions.
Conclusion
In conclusion, hydrogen bonding is a fundamental concept in chemistry that plays a crucial role in many aspects of our daily lives. Understanding the nature and properties of hydrogen bonding is key to comprehending the behavior of molecules and the structure of various compounds. The twelve fascinating facts about hydrogen bonding covered in this article provide a glimpse into the intricate world of chemical bonding. From its role in determining the unique properties of water to its significance in biological structures like DNA, hydrogen bonding offers a wealth of intriguing phenomena to explore.By delving deeper into the mechanisms and effects of hydrogen bonding, scientists can unlock a better understanding of the world around us. From designing new materials to developing life-saving drugs, the implications of hydrogen bonding are vast and significant.So the next time you encounter water’s surface tension, DNA’s double helix, or the unfolding of protein structures, remember that hydrogen bonding lies at the heart of these phenomena. Embrace the wonder of hydrogen bonding and the secrets it holds for the future of chemistry.
FAQs
1. What is hydrogen bonding?
Hydrogen bonding is a type of intermolecular force that occurs between a hydrogen atom in one molecule and a highly electronegative atom, such as oxygen or nitrogen, in another molecule.
2. Why is hydrogen bonding important in chemistry?
Hydrogen bonding is important in chemistry because it influences the physical and chemical properties of molecules. It plays a pivotal role in determining the structure of compounds, influencing biological processes, and affecting the behavior of substances like water.
3. What is the significance of hydrogen bonding in water?
Hydrogen bonding in water is responsible for the unique properties of this compound. These include high surface tension, high boiling and melting points, and the ability to dissolve a wide range of solutes.
4. How does hydrogen bonding contribute to the stability of DNA?
Hydrogen bonding between complementary nitrogenous bases (adenine, thymine, cytosine, and guanine) holds the two strands of DNA together in a double helix structure, contributing to its stability and the replication of genetic information.
5. Can hydrogen bonding occur within a molecule?
Yes, hydrogen bonding can occur within a molecule. This is known as intramolecular hydrogen bonding and can influence the conformation and properties of compounds.
Hydrogen bonding's fascinating facts merely scratch the surface of chemistry's wonders. Supramolecular chemistry unveils nature's incredible self-assembly processes, while inorganic chemistry astonishes with its diverse elements and compounds. Explore more captivating chemical phenomena and expand your understanding of the world around us.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


