If you’re a chemistry enthusiast or simply curious about fascinating chemical compounds, you’re in for a treat! In this article, we’ll delve into the captivating world of thiol, a compound that has numerous mind-blowing facts to offer.
Thiol, also known as a sulfhydryl group, is a functional group composed of a sulfur atom bonded to a hydrogen atom. It is commonly found in organic and biological molecules, playing crucial roles in various biochemical processes. Thiol compounds possess unique properties that make them both intriguing and valuable in different fields of study, including chemistry, biology, and medicine.
Join us as we explore eight mind-blowing facts about thiol. Get ready to be amazed by the wonders of this incredible compound and its remarkable applications. Let’s dive in!
Key Takeaways:
- Thiol, a sulfur-containing compound, is vital in biochemistry, pharmaceuticals, and industrial applications. It’s also responsible for the distinct smells of onions, garlic, wines, and cheeses.
- With its ability to participate in chemical reactions and its diverse medical applications, thiol compounds play a crucial role in various biological and industrial processes, making them an essential component in our everyday lives.
Thiol is a sulfur-containing compound
Thiol, also known as a mercaptan, is a type of organic compound that contains a sulfur atom bonded to a hydrogen atom. The presence of the sulfur-hydrogen bond gives thiols their characteristic odorous and pungent smell.
Thiols play a vital role in biochemistry
Thiols are essential in various biological processes. They are involved in protein structure stabilization, enzyme regulation, and cellular signaling. Additionally, thiols have antioxidant properties, protecting cells from oxidative damage.
Thiol compounds are used in the production of pharmaceuticals
Thiols are widely employed in the pharmaceutical industry. They serve as building blocks for drug synthesis and play a crucial role in the development of many therapeutic compounds. Thiols can also be used as antioxidants in pharmaceutical formulations to enhance stability.
Thiol compounds are responsible for some strong and distinctive aromas
Thiols are known for imparting strong and distinct smells in certain foods and beverages. For example, the presence of thiols is responsible for the characteristic aroma of onions and garlic. In addition, thiols play a role in the aroma of certain types of wines and cheeses.
Thiol compounds have industrial applications
Thiols find applications in various industries. They are commonly used as odorants in natural gas to detect gas leaks by their strong smell. Thiol compounds are also used in the production of polymers, such as rubber, as well as in the synthesis of specialty chemicals.
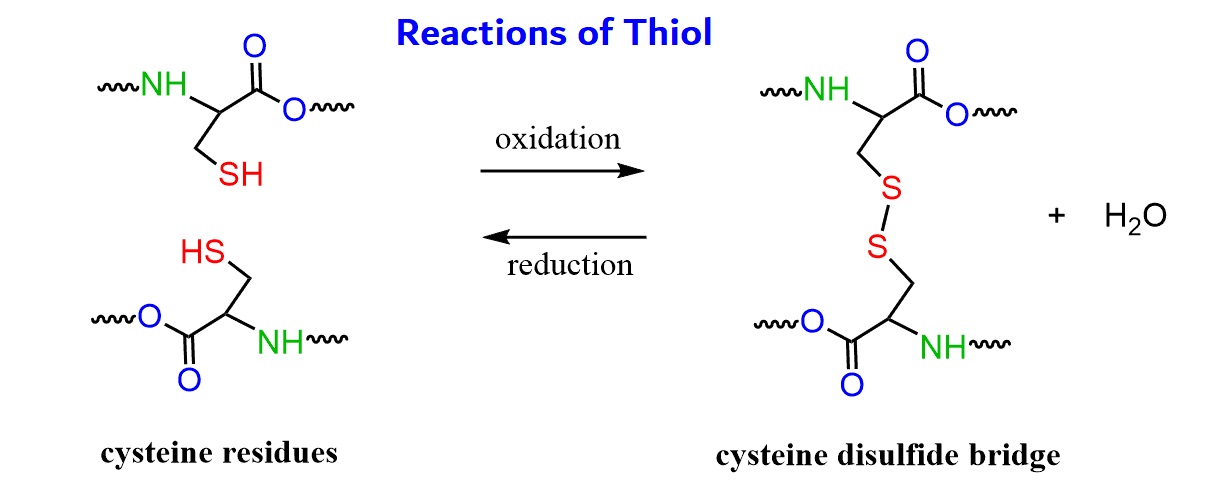
Thiol groups can participate in chemical reactions
The presence of thiol groups in a molecule allows it to undergo various chemical reactions. Thiols can undergo oxidation to form disulfide bonds, which are crucial in protein structure and stability. Thiol groups are also used in thiol-ene reactions, a versatile chemistry for polymer synthesis.
Thiol compounds are used in analytical chemistry
Thiols are widely used in analytical chemistry for their ability to react with metal ions, forming stable complexes. This property makes thiols useful in detecting and quantifying metal ions in environmental samples, such as water and soil.
Thiol compounds have diverse medical applications
Thiols have shown potential in various medical applications. They have been used as antioxidants to protect against oxidative stress-related diseases. Thiol-containing compounds have also been investigated as potential therapeutics for conditions like cardiovascular diseases and cancer.
Conclusion
Thiol is a fascinating compound with a wealth of mind-blowing facts to discover. From its distinct smell to its versatile applications, thiol never fails to surprise. Whether you’re interested in its role in biological systems or its significance in industrial processes, thiol offers a world of exploration and possibilities.
As we have seen, thiol is known for its pungent odor, which can be reminiscent of rotten eggs. This unique smell is due to the sulfur atom present in thiol’s chemical structure. Additionally, thiol plays a crucial role in biological processes, acting as a vital component in the synthesis of proteins and providing protection against oxidative stress.
Moreover, thiol is widely used in various industries. It is utilized as a key ingredient in the production of pharmaceuticals, fragrances, and even as a reducing agent in chemical reactions. Its reactive nature and ability to form strong bonds make it an essential compound in many industrial applications.
Overall, the world of thiol is both intriguing and diverse. Exploring the fascinating facts about this compound opens up a realm of scientific discovery and practical applications.
FAQs
Q: What is thiol?
A: Thiol is a compound that contains a sulfur atom bonded to a hydrogen atom. It is also known as a sulfhydryl group (-SH) and is characterized by its pungent odor.
Q: Where can thiol be found?
A: Thiol can be found in various natural sources such as garlic, onions, and durian fruit. It is also a component of many organic molecules and is widely used in industries.
Q: What gives thiol its distinctive smell?
A: The distinctive smell of thiol is attributed to the presence of the sulfur atom in its structure. This sulfur atom produces a distinct odor, often described as similar to that of rotten eggs.
Q: What are the applications of thiol?
A: Thiol has numerous applications, ranging from its use in pharmaceuticals and fragrances to its role as a reducing agent in chemical reactions. It is also an important component in biological systems, playing a vital role in protein synthesis and protection against oxidative stress.
Q: Is thiol dangerous?
A: Thiol can be toxic in high concentrations. Therefore, it should be handled with care and proper safety precautions should be followed when working with this compound.
Thiol's fascinating properties and diverse applications make it a captivating subject for those interested in chemistry and its real-world implications. Thiols' importance in biochemistry, particularly their role in protein structure and function, highlights their significance in living organisms. Furthermore, thiol compounds' use in pharmaceutical production and their distinctive aromas demonstrate their practical applications. For those curious about the chemical world, exploring the wonders of biochemistry and the intricacies of redox reactions can provide even more mind-blowing facts and insights into the complex and captivating realm of chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


