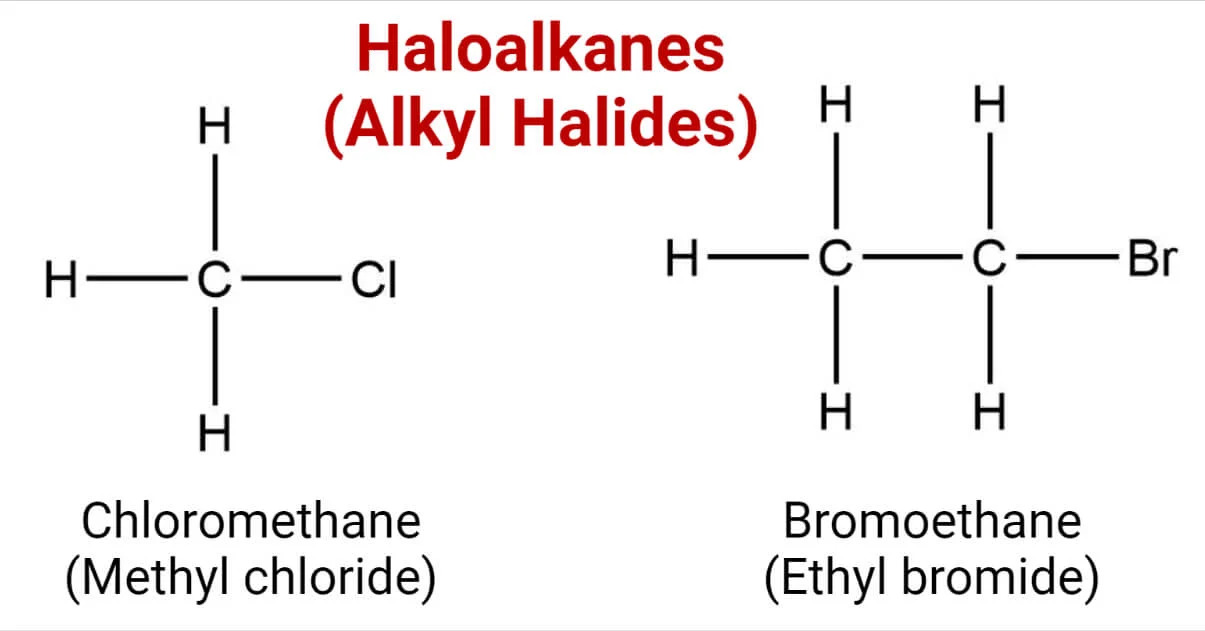
Alkyl halides, also known as haloalkanes, are a fascinating class of organic compounds that have played a significant role in the field of chemistry. These compounds are characterized by the presence of a halogen atom (such as fluorine, chlorine, bromine, or iodine) attached to a carbon atom. While they may seem simple at first glance, alkyl halides have a wide range of applications and properties that make them truly remarkable.
In this article, we are going to explore 20 unbelievable facts about alkyl halides that will deepen your understanding of these compounds. From their unique reactivity to their diverse applications in industrial processes and pharmaceuticals, alkyl halides have a rich history and continue to be an area of active research for chemists across the globe.
Key Takeaways:
- Alkyl halides are versatile organic compounds widely used in industries like pharmaceuticals and plastics due to their reactivity and unique chemical properties.
- Some alkyl halides can be toxic and have environmental concerns, such as contributing to ozone depletion, highlighting the importance of proper handling and disposal.
Alkyl Halides Are Organic Compounds
Alkyl halides are a type of organic compound that contain halogen atoms, such as fluorine, chlorine, bromine, or iodine, bonded to an alkyl group.
Alkyl Halides Are Widely Used in Industrial Applications
Due to their unique chemical properties, alkyl halides find numerous applications in industries such as pharmaceuticals, agrochemicals, plastics, and solvents.
Alkyl Halides Are Highly Reactive
Alkyl halides exhibit reactivity due to the presence of halogen atoms, making them susceptible to various types of chemical reactions.
Alkyl Halides Can Serve as Starting Materials for Organic Synthesis
Due to their reactivity and availability, alkyl halides are commonly used as starting materials in organic synthesis processes to create more complex organic compounds.
Alkyl Halides Can Undergo Substitution Reactions
One of the most common types of reactions observed in alkyl halides is substitution, where the halogen atom is replaced by another group.
Alkyl Halides Are Used as Intermediates in Pharmaceutical Synthesis
Alkyl halides play a crucial role as intermediates in the synthesis of various pharmaceutical drugs. Their reactivity allows for the introduction of specific functional groups into the desired organic molecule.
Alkyl Halides Can Act as Nucleophiles or Electrophiles
Depending on the reaction conditions, alkyl halides can either act as nucleophiles by donating electrons or as electrophiles by accepting electrons.
Alkyl Halides Can Undergo Elimination Reactions
Besides substitution, alkyl halides can also undergo elimination reactions where a halogen atom is eliminated along with an adjacent hydrogen atom to form a double bond.
Alkyl Halides Are Used in the Production of Plastics
Many types of plastics, including polyvinyl chloride (PVC), are produced using alkyl halides as starting materials.
Alkyl Halides Have Diverse Physical Properties
The physical properties of alkyl halides vary according to the size and nature of the alkyl group as well as the type of halogen atom present.
Alkyl Halides Can Exhibit Chirality
When the carbon atom bonded to the halogen is attached to four different substituents, alkyl halides can exist as enantiomers and display optical activity.
Alkyl Halides Can Undergo Radical Reactions
Under specific conditions, alkyl halides can undergo radical reactions where a halogen atom is replaced by a radical group.
Alkyl Halides Are Used in Pesticide Production
Certain alkyl halides find application in the production of pesticides, where their reactivity aids in the synthesis of effective crop protection chemicals.
Alkyl Halides Can Be Toxic
Some alkyl halides are known to be toxic and can cause harm to humans and the environment. Proper handling and disposal are essential.
Alkyl Halides Are Insoluble in Water
Most alkyl halides have low solubility in water, due to their nonpolar nature. This property makes them suitable for use in organic solvents.
Alkyl Halides Can Be Derived from Alcohols
Alkyl halides can be synthesized by reacting alcohols with hydrogen halides or by using other halogenating agents.
Alkyl Halides Are Used in Flame Retardants
Due to their fire-resistant properties, certain alkyl halides are used as flame retardants in various applications including electronics and textiles.
Alkyl Halides Can Undergo Rearrangement Reactions
Under specific conditions, alkyl halides can undergo rearrangement reactions where the carbon skeleton is rearranged to form a more stable product.
Alkyl Halides Can Participate in Cross-Coupling Reactions
Alkyl halides are often used in cross-coupling reactions, where two different alkyl groups are joined together to form a more complex organic structure.
Alkyl Halides Have Environmental Concerns
Some alkyl halides, such as chlorofluorocarbons (CFCs), have been found to contribute to ozone depletion and are now regulated in many countries.
Conclusion
In conclusion, alkyl halides are fascinating compounds with a wide range of applications and properties. From their role in organic synthesis to their importance in environmental and biological processes, alkyl halides play a significant role in the field of chemistry. Understanding their unique characteristics, reactivity, and various reactions can help scientists develop new drugs, improve industrial processes, and contribute to advancements in multiple scientific disciplines.
By delving into the 20 unbelievable facts about alkyl halides, we have explored the diverse world of these compounds. From their impact on the ozone layer to their use in flame retardants and as anesthetics, alkyl halides have both positive and negative influences.
As advances in chemistry continue to push boundaries, the knowledge gained about alkyl halides will undoubtedly contribute to the development of new materials, medicines, and technologies. It is truly remarkable how the study of these compounds opens up countless possibilities for improving our lives and the world around us.
FAQs
Q: What are alkyl halides?
A: Alkyl halides are organic compounds that contain a halogen atom bonded to an alkyl group. They are often used as starting materials in organic synthesis.
Q: What are some common examples of alkyl halides?
A: Some common examples of alkyl halides include chloroform (CHCl3), bromomethane (CH3Br), and fluoromethane (CH3F).
Q: How are alkyl halides used in industry?
A: Alkyl halides are used in various industrial applications such as pharmaceutical manufacturing, agrochemicals production, and the production of plastics and solvents.
Q: Are alkyl halides harmful to the environment?
A: Some alkyl halides, particularly those containing chlorine and bromine, have been found to be harmful to the environment as they contribute to the depletion of the ozone layer.
Q: Can alkyl halides be used as anesthetics?
A: Yes, certain alkyl halides such as chloroform and halothane have been used as anesthetics due to their ability to produce a state of unconsciousness during medical procedures.
Q: How do alkyl halides participate in nucleophilic substitution reactions?
A: Alkyl halides undergo nucleophilic substitution reactions where the halogen atom is replaced by a nucleophile, resulting in the formation of a new compound.
Q: Are alkyl halides water-soluble?
A: Generally, alkyl halides have low water solubility due to the presence of the non-polar alkyl group. However, their solubility can vary depending on the size and polarity of the halogen atom.
Alkyl halides' fascinating properties and wide-ranging applications make them a captivating topic for chemistry enthusiasts. Dive deeper into the world of organic chemistry by exploring halogenation, a process that introduces halogens to compounds. Uncover the intricacies of nucleophilic substitution reactions, where alkyl halides play a crucial role. Grignard reagents, derived from alkyl halides, offer a wealth of synthetic possibilities. Embark on a journey through these enthralling subjects and expand your understanding of alkyl halides' significance in chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.