If you’ve ever taken a chemistry class, you’ve likely come across the concept of activation energy. It’s a fundamental concept that plays a crucial role in understanding chemical reactions and kinetics. Activation energy refers to the minimum amount of energy required for a reaction to occur. But there’s more to activation energy than meets the eye. In this article, we will explore 18 extraordinary facts about activation energy that will deepen your understanding of this essential concept. From its impact on reaction rates to its relation to catalysts, these facts will shed light on the fascinating world of activation energy and its significance in the field of chemistry. So let’s dive in and discover the wonders of activation energy!
Key Takeaways:
- Activation energy is the energy needed to start a chemical reaction. It determines how fast a reaction happens. Lower activation energy means faster reactions, while higher activation energy means slower reactions.
- Catalysts help lower activation energy, making reactions happen faster. Understanding activation energy is important for industrial processes and sustainable chemistry to improve efficiency and reduce environmental impact.
What is Activation Energy?
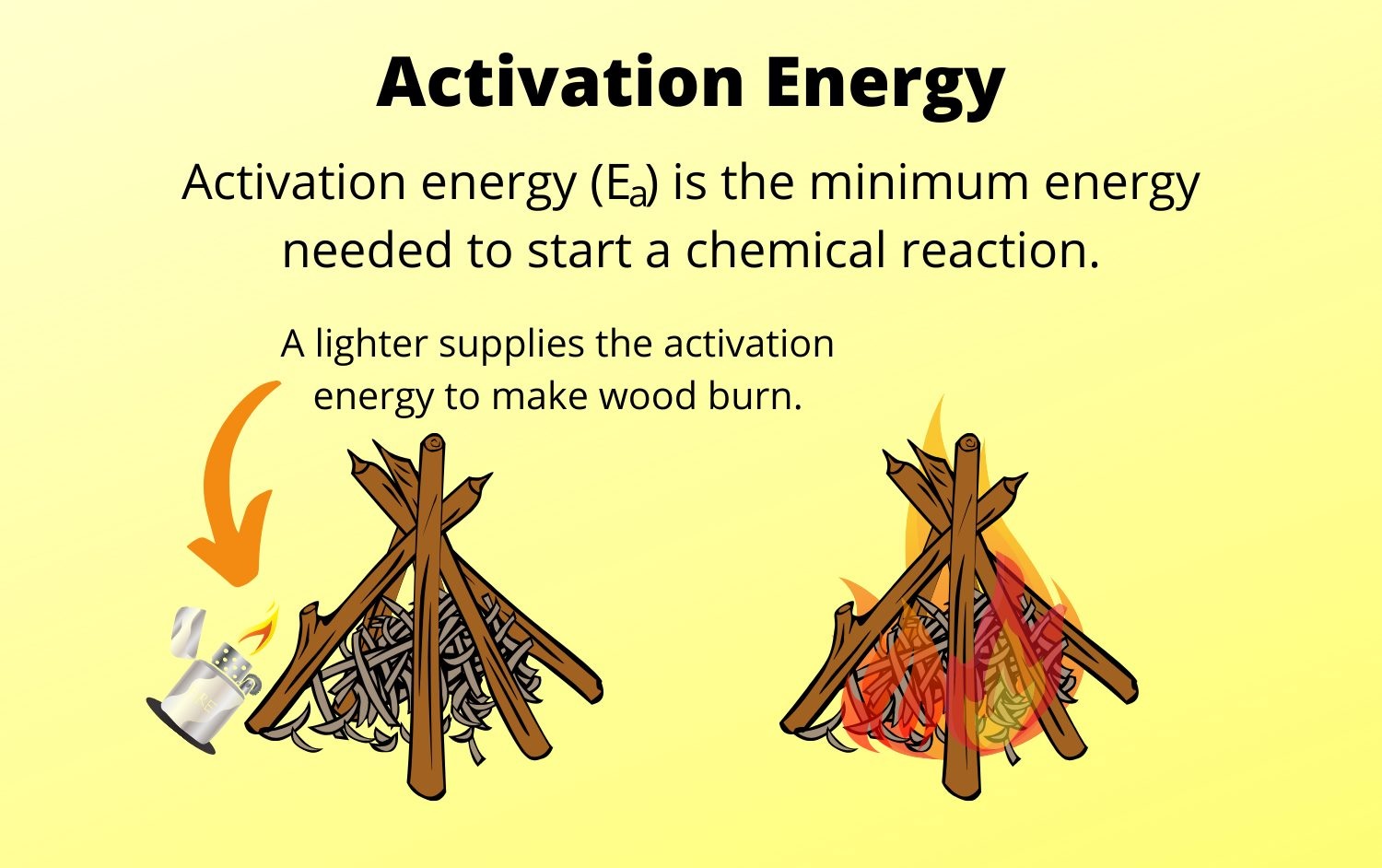
Activation energy is the minimum amount of energy required for a chemical reaction to occur. It is the energy barrier that must be overcome for the reactants to transform into products.
Importance of Activation Energy
Activation energy plays a crucial role in determining the rate of a chemical reaction. Higher activation energy leads to slower reactions, while lower activation energy leads to faster reactions.
Activation Energy and Reaction Rates
The relationship between activation energy and reaction rates is governed by the Arrhenius equation. According to this equation, an increase in activation energy leads to a decrease in the reaction rate.
Catalysts and Activation Energy
Catalysts are substances that lower the activation energy of a chemical reaction without being consumed in the process. They provide an alternative reaction pathway with lower energy requirements.
Activation Energy and Temperature
An increase in temperature generally leads to a decrease in activation energy. This is because higher temperatures provide more energy to the reactant molecules, allowing them to overcome the energy barrier more easily.
Collision Theory and Activation Energy
The collision theory states that for a reaction to occur, the reactant molecules must collide with sufficient energy and proper orientation. Activation energy determines whether the collision is successful in producing products.
Transition State Theory
Transition state theory describes the intermediate state that forms during a chemical reaction, known as the activated complex. Activation energy is required for the reactants to reach this transition state and proceed to form products.
Activation Energy and Reaction Mechanisms
Activation energy influences the reaction mechanism, which is the sequence of elementary steps by which a reaction occurs. Different reaction mechanisms have different activation energies and determine the overall rate of the reaction.
Activation Energy and Biological Reactions
Activation energy plays a vital role in biological reactions. Enzymes act as biological catalysts, lowering the activation energy required for biochemical reactions to take place within living organisms.
Energy Diagrams and Activation Energy
Energy diagrams, also known as reaction coordinate diagrams, visualize the energy changes that occur during a chemical reaction. Activation energy is represented by the energy barrier between reactants and products.
Factors Affecting Activation Energy
Several factors can influence the activation energy of a reaction, including the nature of the reactants, concentration, pressure, and the presence of a catalyst.
Activation Energy and Exothermic Reactions
In exothermic reactions, the reactants possess more energy than the products. Activation energy is required to start the reaction and release the excess energy, resulting in a decrease in overall energy.
Activation Energy and Endothermic Reactions
Endothermic reactions require energy input to proceed. Activation energy in endothermic reactions is higher than the energy released during the reaction to compensate for the energy absorbed.
Activation Energy and Chemical Equilibrium
Activation energy influences the position of chemical equilibrium. Higher activation energy favors the formation of products, while lower activation energy favors the formation of reactants.
Activation Energy and Reactant Stability
Reactant stability influences the activation energy required for a reaction. More stable reactants typically have higher activation energies, making the reaction less likely to occur spontaneously.
Activation Energy and Industrial Applications
Understanding activation energy is crucial in industrial processes, such as chemical manufacturing and refining. By optimizing the activation energy, companies can improve reaction efficiency and reduce costs.
Activation Energy and Rate-Determining Steps
In complex reactions, the step with the highest activation energy is often the rate-determining step. This step controls the overall rate of the reaction and determines how quickly products are formed.
Activation Energy and Sustainable Chemistry
Sustainable chemistry aims to develop processes with lower activation energies, reducing energy consumption and environmental impact. This field focuses on designing catalysts and reaction conditions to minimize activation energy requirements.
Conclusion
Activation energy is a fascinating and significant concept in the field of chemistry. Hopefully, this article has shed some light on its importance and provided you with 18 extraordinary facts about activation energy. From its role in chemical reactions to its impact on reaction rates, activation energy plays a crucial role in understanding the behavior of substances at a molecular level.Understanding activation energy can help chemists design more efficient reactions, develop new catalysts, and optimize reaction conditions. As we delve deeper into the world of chemistry, we continue to uncover new insights into activation energy and its implications.By grasping the concept of activation energy, we can unlock the secrets of chemical reactions and better understand how nature and the molecules within it function. So, dive into this realm of chemistry, explore the intricate world of activation energy, and uncover the marvels that it holds.
FAQs
1. What is activation energy?
Activation energy is the minimum amount of energy required for a chemical reaction to occur. It is the energy barrier that must be overcome for reactants to transform into products.
2. How does activation energy affect reaction rates?
Higher activation energy leads to slower reaction rates, as more energy is needed to initiate the reaction. Lower activation energy, on the other hand, enhances the reaction rate by requiring less energy for reactants to turn into products.
3. Can activation energy be altered?
Yes, activation energy can be altered by factors such as temperature, catalysts, and the concentration of reactants. Increasing temperature and using catalysts can lower the activation energy and accelerate the reaction rate.
4. What is the significance of activation energy in chemical reactions?
Activation energy determines the feasibility and speed of a chemical reaction. It provides valuable insights into the energy requirements and pathways involved in the transformation of reactants to products.
5. How can activation energy be calculated or measured?
Activation energy can be calculated using various methods, such as the Arrhenius equation or transition state theory. Experimental techniques like differential scanning calorimetry (DSC) and kinetic studies can also be used to measure activation energy.
6. Are there any practical applications of activation energy?
Understanding activation energy is crucial in fields such as chemical engineering, pharmaceuticals, and materials science. It helps in designing efficient processes, developing new drugs, and optimizing the performance of materials.
7. Does every chemical reaction have activation energy?
Yes, every chemical reaction has activation energy, as it is a fundamental requirement for a reaction to occur. However, the magnitude of activation energy can vary depending on the specific reaction and its conditions.
8. Can activation energy be zero?
No, activation energy cannot be zero. Even reactions that appear spontaneous still require a minimum amount of energy to overcome the energy barrier between reactants and products.
Activation energy plays a critical role in chemical reactions, influencing reaction rates and shaping the world of chemistry. Understanding this concept provides valuable insights into how reactions occur and can be controlled. If you're curious to learn more about related topics, explore the fascinating realm of kinetics, which studies reaction rates and mechanisms. Additionally, delve into the astonishing world of transition states, the fleeting intermediates formed during chemical reactions. By grasping these fundamental concepts, you'll gain a deeper appreciation for the complex and captivating nature of chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
