Reaction intermediates are an essential component in understanding the intricacies of chemical reactions. These elusive species play a crucial role in the conversion of reactants to products, acting as intermediaries between the starting materials and the final outcome. While reaction intermediates are transient and short-lived, studying them can provide invaluable insights into reaction mechanisms and help scientists gain a deeper understanding of chemical processes.
In this article, we will explore 20 surprising facts about reaction intermediates that will fascinate both chemistry enthusiasts and professionals. From their existence in multiple reaction types to their role in catalysis and their influence on reaction rates, we will delve into the intriguing world of these often-overlooked chemical entities. So let’s dive in and uncover the surprising secrets held by reaction intermediates!
Key Takeaways:
- Reaction intermediates are like backstage helpers in a chemical reaction, bridging the gap between reactants and products, and their study helps scientists unlock the secrets of complex reactions.
- These transient species, with their high energy and unique properties, hold the key to creating new molecules and understanding the intricate dance of atoms in the world of chemistry.
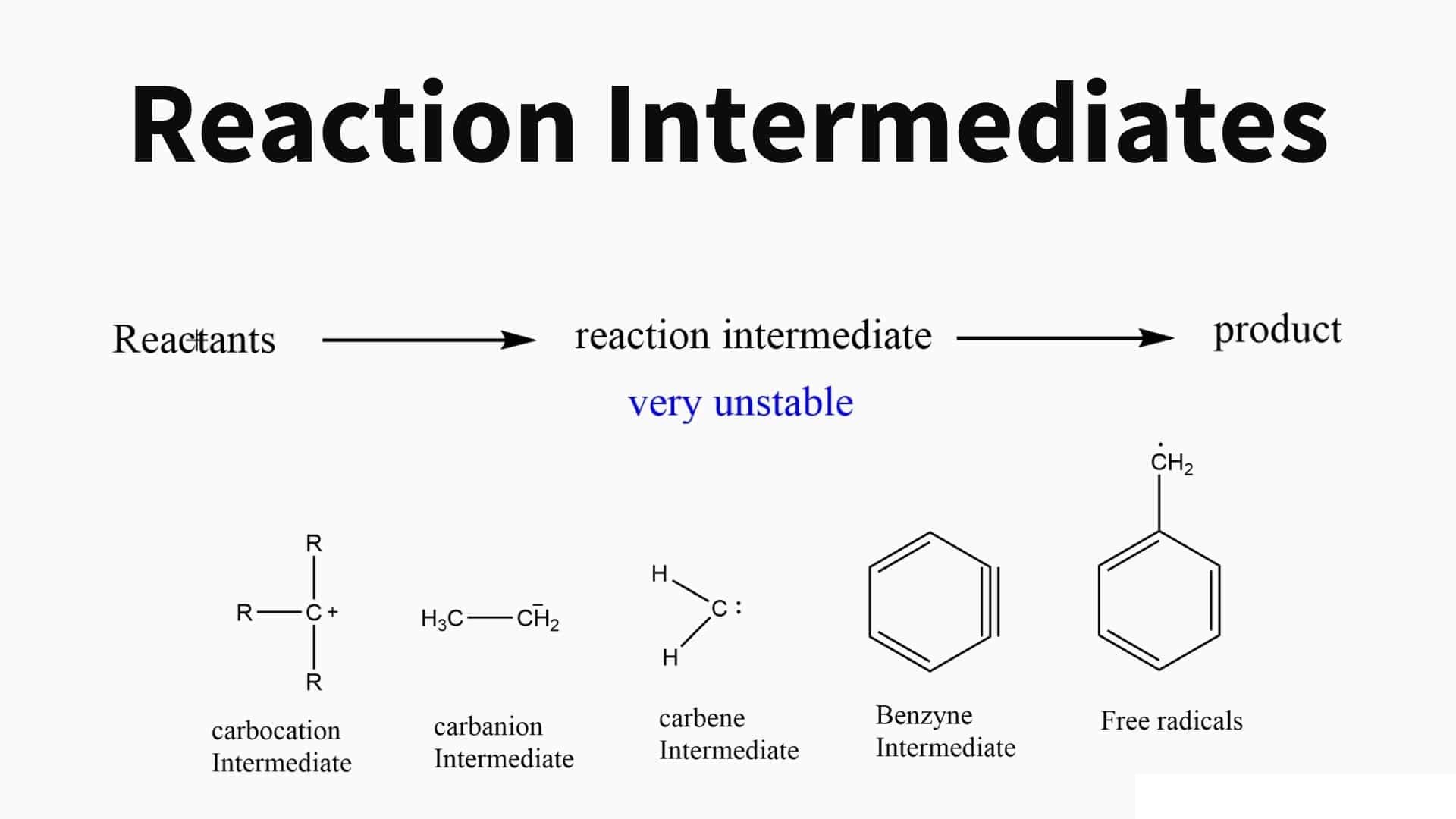
Reaction intermediates play a crucial role in chemical reactions.
A reaction intermediate is a chemical species that forms during the course of a reaction and is consumed in a subsequent step. It acts as a bridge between the reactants and the final products, helping to facilitate the reaction process.
Reaction intermediates can exist in various forms.
Reaction intermediates can be ions, radicals, or molecules that possess high energy and unstable characteristics. These transient species are often short-lived and undergo further reactions to yield the desired products.
The formation of reaction intermediates is often influenced by reaction conditions.
Factors such as temperature, pressure, catalysts, and concentration of reactants can significantly impact the generation and stability of reaction intermediates. Manipulating these conditions can alter the reaction pathway and lead to different outcomes.
Reaction intermediates can provide valuable insights into reaction mechanisms.
Studying reaction intermediates allows chemists to understand the step-by-step progression of a chemical reaction. By identifying and characterizing these species, scientists can decipher the intricacies of complex reactions and develop more efficient strategies for synthesis.
Transition states are a type of reaction intermediate.
Transition states are high-energy species that exist temporarily during a reaction. They represent the highest energy point along the reaction pathway and are characterized by partial bonds and distorted molecular geometries.
Reaction intermediates can be detected and analyzed using spectroscopic techniques.
Various spectroscopic methods, such as infrared spectroscopy, nuclear magnetic resonance (NMR), and mass spectrometry, can provide valuable information about the structure, composition, and behavior of reaction intermediates.
Some reaction intermediates have unique reactivity and selectivity.
Certain intermediates exhibit specific reactivity towards certain functional groups or classes of compounds. This selectivity can be harnessed in organic synthesis to achieve highly specific and controlled transformations.
Radicals are common reaction intermediates.
Radicals are highly reactive species that possess unpaired electrons. They often initiate and propagate chain reactions, playing a crucial role in many organic and polymerization reactions.
Reaction intermediates can be stabilized through coordination with catalysts.
Catalysts can interact with reaction intermediates, modifying their electronic properties and stabilizing them. This allows for the formation of new bonds and accelerates the overall reaction rate.
Acid-base reactions often involve reaction intermediates.
Proton transfer reactions, commonly known as acid-base reactions, frequently involve the formation of intermediate species. These intermediates act as transient carriers of the proton before being further transformed into the final products.
Reaction intermediates can undergo rearrangements.
During the course of a reaction, intermediates may undergo structural rearrangements, leading to the formation of different products. These rearrangements can occur through migration of atoms or groups within the intermediate molecule.
Reaction intermediates can be isolable under certain conditions.
In some cases, reaction intermediates can be trapped or selectively formed under specific reaction conditions, allowing for their isolation and characterization. This provides a deeper understanding of their properties and behavior.
Some reaction intermediates have unique electronic configurations.
Due to their high energy and unstable nature, reaction intermediates often exhibit unusual electronic structures, such as radical character or partial charges. These electronic features contribute to their distinct reactivity.
Reaction intermediates can undergo multiple reaction pathways.
Depending on the reaction conditions and the nature of the reactants, reaction intermediates may follow different pathways leading to different products. Understanding the factors that control these pathways is essential for reaction optimization.
Some reaction intermediates act as catalysts themselves.
In certain reactions, the reaction intermediate can act as a catalyst, facilitating the conversion of reactants into products. This phenomenon, known as autocatalysis, can lead to self-amplifying reaction cycles.
Organic reactions often involve reaction intermediates.
Organic chemistry relies heavily on reaction intermediates, as they play a crucial role in the synthesis of complex organic molecules. Understanding the behavior of intermediates is key to designing efficient synthetic routes.
Reaction intermediates are influenced by solvent effects.
The choice of solvent can have a significant impact on the stability and reactivity of reaction intermediates. Different solvents can solvate the intermediate species differently, leading to variations in the reaction rate and selectivity.
Reaction intermediates can be formed through radical reactions.
Radical reactions involve the generation of reactive intermediates called radicals. These species have unpaired electrons and exhibit unique reactivity, allowing for the formation of diverse products.
Some reaction intermediates have biological significance.
In biochemical reactions, reaction intermediates play crucial roles in the synthesis of biomolecules and the functioning of enzymes. Understanding these intermediates is essential for unraveling biological processes.
Reaction intermediates are key to understanding complex reaction networks.
Many chemical reactions occur through intricate networks of intermediates and transition states. Analyzing the behavior and interactions of these intermediates provides valuable insight into the overall reaction mechanism.
Conclusion
In conclusion, reaction intermediates play a crucial role in chemical reactions. They are short-lived species that form during the course of a reaction and serve as a stepping stone between reactants and products. While some reaction intermediates are well-known and extensively studied, there are many surprising facts about them that are still being uncovered.Throughout this article, we have explored 20 surprising facts about reaction intermediates. We have learned that reaction intermediates can exist in different states, such as ions, free radicals, or complexes. They can also be stable or highly reactive, depending on the specific reaction.Furthermore, it has been fascinating to discover how reaction intermediates can be used to explain reaction mechanisms, unveil new synthetic routes, and even provide insights into biological processes. The study of reaction intermediates continues to push the boundaries of chemistry and contributes to the development of new technologies and materials.In summary, reaction intermediates are a dynamic and intriguing aspect of chemistry. By further understanding their properties and behavior, scientists can unlock new possibilities in the world of chemical reactions.
FAQs
Q: What is a reaction intermediate?
A: A reaction intermediate is a short-lived species that forms during a chemical reaction and exists between the initial reactants and final products.
Q: How are reaction intermediates detected?
A: Reaction intermediates can be detected through various techniques such as spectroscopy, chromatography, and computational methods.
Q: Why are reaction intermediates important?
A: Reaction intermediates are important because they help explain reaction mechanisms, guide the development of new synthetic routes, and provide insights into biological processes.
Q: Can reaction intermediates be isolated and studied?
A: In some cases, reaction intermediates can be isolated and studied using specialized techniques. However, due to their highly reactive nature, many intermediates are difficult to isolate and study directly.
Q: Are all reactions accompanied by the formation of reaction intermediates?
A: Not all reactions involve the formation of reaction intermediates. Some reactions proceed through a single-step mechanism, while others involve the formation of multiple intermediates.
Q: Can reaction intermediates impact the selectivity of a reaction?
A: Yes, reaction intermediates can influence the selectivity of a reaction by favoring the formation of certain products over others. Understanding the nature of intermediates can provide insights into controlling reaction outcomes.
Q: Can reaction intermediates be utilized in practical applications?
A: Absolutely! Reaction intermediates can be harnessed for various practical applications, such as developing new catalysts, designing more efficient chemical processes, and synthesizing complex molecules.
Reaction intermediates hold countless secrets waiting to be unveiled. Their fleeting existence and critical role in chemical transformations make them a captivating subject for scientists and curious minds alike. If you found these 20 surprising facts about reaction intermediates intriguing, prepare to be amazed by even more fascinating insights. Explore the hidden world of reaction intermediates and their impact on our understanding of chemical reactions. Unravel the mysteries surrounding these elusive species and gain a deeper appreciation for the complexity of chemical processes. Get ready to embark on a journey of discovery as you delve into the captivating realm of reaction intermediates.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


