Infrared spectroscopy, commonly known as IR spectroscopy, is an incredibly valuable tool in the field of chemistry. It allows scientists to gain insights into the composition and structure of compounds by analyzing their interaction with infrared light. While many of us might be familiar with visible light and its role in our daily lives, the invisible realm of infrared radiation opens up a whole new world of possibilities in the realm of analytical chemistry.
In this article, we will dive into the fascinating world of infrared spectroscopy and explore eight enigmatic facts that make it such a vital tool in the study of molecular compounds. Whether you are a chemistry enthusiast or simply curious about the science behind IR spectroscopy, these intriguing facts will give you a deeper understanding of this essential technique. So, let’s unlock the secrets of infrared spectroscopy and unravel the mysteries it holds!
Key Takeaways:
- Infrared Spectroscopy (IR) uses invisible light to uncover the unique vibrations of molecules, helping scientists identify substances and understand molecular structures.
- IR spectroscopy is non-destructive, making it valuable for analyzing delicate materials, and has diverse applications in industries such as pharmaceuticals, forensics, and environmental monitoring.
The Invisible Light: What is Infrared Radiation?
IR spectroscopy deals with a region of the electromagnetic spectrum that lies beyond the visible light spectrum. It encompasses wavelengths longer than those of visible light, with frequencies ranging from 300 GHz to 400 THz. This invisible light enables us to observe the molecular vibrations that are characteristic of different types of chemical bonds.
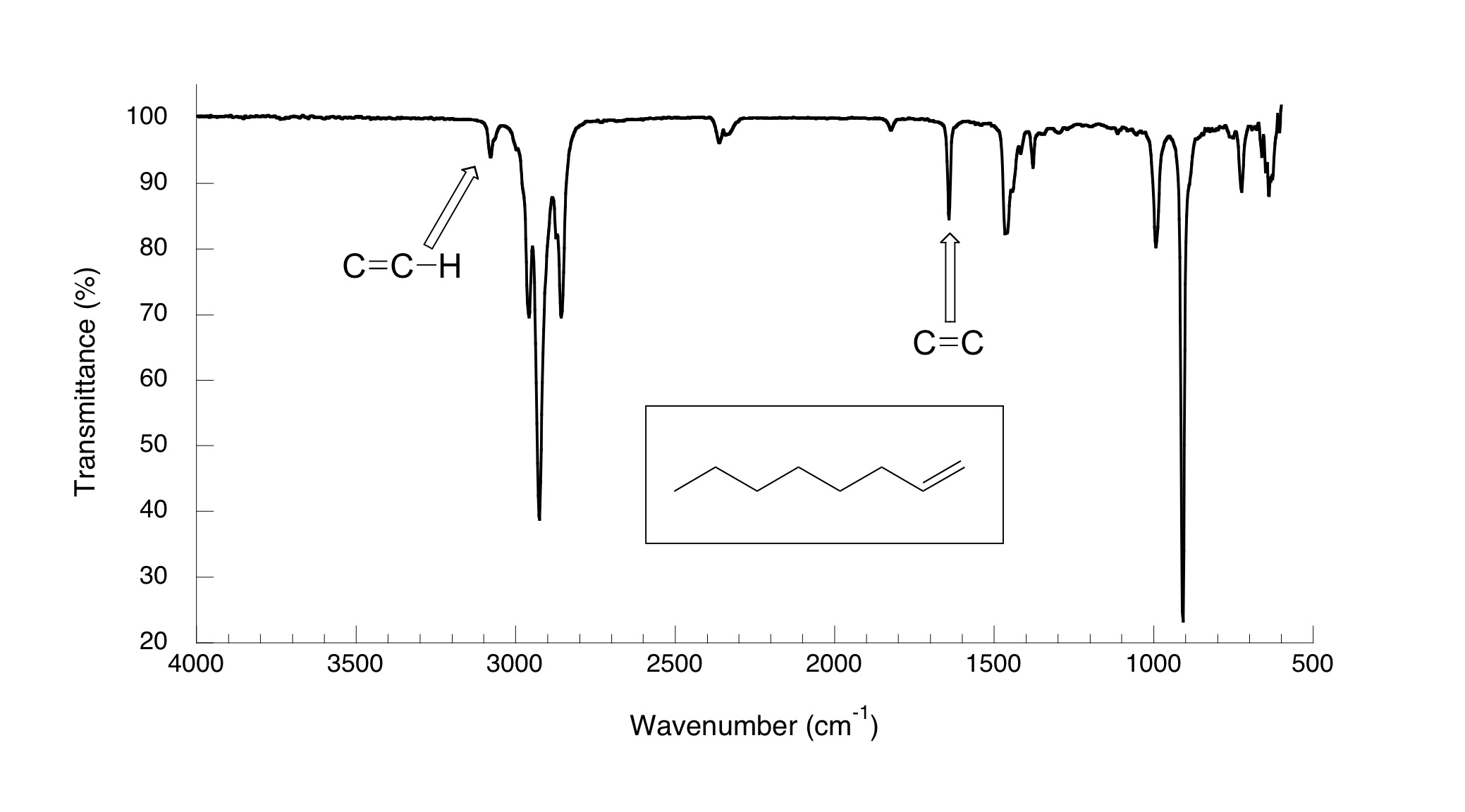
Molecular Fingerprints: Identifying Substances
IR spectroscopy provides unique molecular fingerprints, allowing chemists to identify and differentiate substances. Every chemical compound possesses its own distinct set of absorption peaks in the IR spectrum, enabling accurate identification and characterization of unknown samples.
The Stretch and Bend: Understanding Molecular Vibrations
Molecules vibrate in specific ways, with characteristic stretching and bending motions. IR spectroscopy focuses on analyzing these vibrational modes. When infrared light interacts with a molecule, it causes the bonds to stretch or bend, resulting in absorption of specific wavelengths of light. By studying these vibrational frequencies, scientists can determine the types of bonds present in a compound.
Quantitative Analysis: Determining Concentrations
In addition to qualitative analysis, IR spectroscopy can also be used for quantitative analysis. By measuring the intensity of absorption at specific wavelengths, scientists can determine the concentration of a component within a mixture. This makes IR spectroscopy a valuable technique in pharmaceutical, environmental, and forensic applications.
Polymorphism: Investigating Crystal Structures
IR spectroscopy plays a significant role in studying polymorphism, the ability of a substance to exist in multiple crystal structures. Different crystal forms can possess distinctive IR spectra, aiding in the identification and characterization of polymorphic compounds.
Non-destructive: Analyzing Intact Samples
One of the major advantages of IR spectroscopy is its non-destructive nature. Samples can be analyzed without undergoing any chemical changes or requiring extensive preparation. This makes it a valuable tool for studying delicate or precious materials, such as artworks or archaeological artifacts.
Chemical Imaging: Mapping Spatial Distribution
IR spectroscopy can be combined with advanced imaging techniques to create chemical maps of samples. This allows scientists to visualize and analyze the spatial distribution of different chemical components within a sample, providing valuable insights into the composition and structure of complex materials.
Industry Applications: From Pharmaceuticals to Forensics
IR spectroscopy finds numerous applications in various industries. It is used in pharmaceutical development to analyze drug formulations, assess purity, and detect impurities. In forensic analysis, it helps identify unknown substances found at crime scenes. Additionally, IR spectroscopy is employed in environmental monitoring, food analysis, and materials science.
With its ability to unlock the hidden world of molecules, Infrared Spectroscopy (IR) continues to be an invaluable analytical technique. By understanding these eight enigmatic facts about IR spectroscopy, we gain a deeper appreciation for its importance in unraveling the mysteries of chemistry.
Conclusion
Infrared spectroscopy (IR) is a fascinating and powerful analytical technique that has revolutionized the field of chemistry. Through the analysis of the interaction between matter and infrared radiation, IR spectroscopy provides valuable insights into the molecular structure and chemical composition of a wide range of substances.Throughout this article, we have explored eight enigmatic facts about infrared spectroscopy that showcase its significance in various applications. From identifying functional groups and determining the purity of compounds to studying the mechanisms of chemical reactions, IR spectroscopy offers immense potential in solving complex chemical problems.Understanding the principles and applications of infrared spectroscopy is not only crucial for chemists and researchers but also relevant to industries such as pharmaceuticals, forensics, and environmental analysis. By harnessing the power of IR spectroscopy, scientists can unravel the mysteries of matter and unlock new possibilities for innovation.In conclusion, the world of infrared spectroscopy is both captivating and essential in the realm of chemistry. Its ability to unravel the molecular structure of compounds and provide valuable chemical information makes it a vital tool for researchers across various disciplines.
FAQs
Q: What is infrared spectroscopy?
A: Infrared spectroscopy is a technique used to analyze how molecules interact with infrared radiation, providing insights into the molecular structure and chemical composition of substances.
Q: How is infrared spectroscopy used in chemistry?
A: Infrared spectroscopy is commonly used in chemistry to identify functional groups, determine the purity of compounds, study reaction mechanisms, and analyze various types of samples.
Q: What are some practical applications of infrared spectroscopy?
A: Infrared spectroscopy finds applications in a wide range of fields, including pharmaceutical analysis, environmental monitoring, forensic investigations, food analysis, and materials science.
Q: How does infrared spectroscopy work?
A: Infrared spectroscopy works by passing infrared radiation through a sample and measuring the absorption of specific wavelengths, which corresponds to the vibrational frequencies of molecular bonds.
Q: Can infrared spectroscopy determine the structure of unknown compounds?
A: Yes, infrared spectroscopy can provide valuable information about the functional groups present in a compound, helping in the identification and partially determining the structure of unknown compounds.
Q: Are there any limitations to the use of infrared spectroscopy?
A: Yes, while infrared spectroscopy is a powerful technique, it has some limitations. It cannot provide information about the spatial arrangement of molecules or distinguish between optical isomers.
Q: What are the different types of infrared spectroscopy?
A: The different types of infrared spectroscopy include Fourier transform infrared (FTIR) spectroscopy, near-infrared (NIR) spectroscopy, and attenuated total reflectance (ATR) spectroscopy.
Q: Is infrared spectroscopy a non-destructive technique?
A: Infrared spectroscopy is often considered a non-destructive technique as it generally requires very small sample amounts and leaves the sample intact for further analysis.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
