The wave function is a fundamental concept in quantum mechanics that describes the state of a particle or system. It plays a crucial role in understanding the behavior and properties of subatomic particles, leading to mind-blowing discoveries and phenomena. In this article, we will dive into the intriguing world of wave function and explore 12 fascinating facts that will surely leave you in awe. From the wave-particle duality to the uncertainty principle, these facts will challenge your perception of reality and illuminate the bizarre nature of the quantum realm. So, fasten your seatbelts and get ready for a mind-bending journey into the mysterious realm of the wave function!
Key Takeaways:
- Wave function is like a quantum crystal ball, showing us the chances of finding particles in different places or states. It’s like predicting where a friend might be at a party!
- Quantum superposition is like a particle’s magic trick, being in multiple states at once. The wave function helps us calculate the probabilities of these mind-boggling states.
Wave Function is a Probability Distribution
The wave function represents the probability distribution of a particle’s position or state in quantum mechanics. It describes the likelihood of finding the particle at a particular location or having specific properties.
Wave Function Describes Quantum Superposition
Quantum superposition is a phenomenon where particles can exist in multiple states simultaneously. The wave function allows us to calculate the probabilities of these different states and their combinations.
Wave Function in Quantum Interference
Quantum interference occurs when two or more wave functions overlap, resulting in constructive or destructive interference patterns. This phenomenon is essential in understanding the behavior of particles such as electrons and photons.
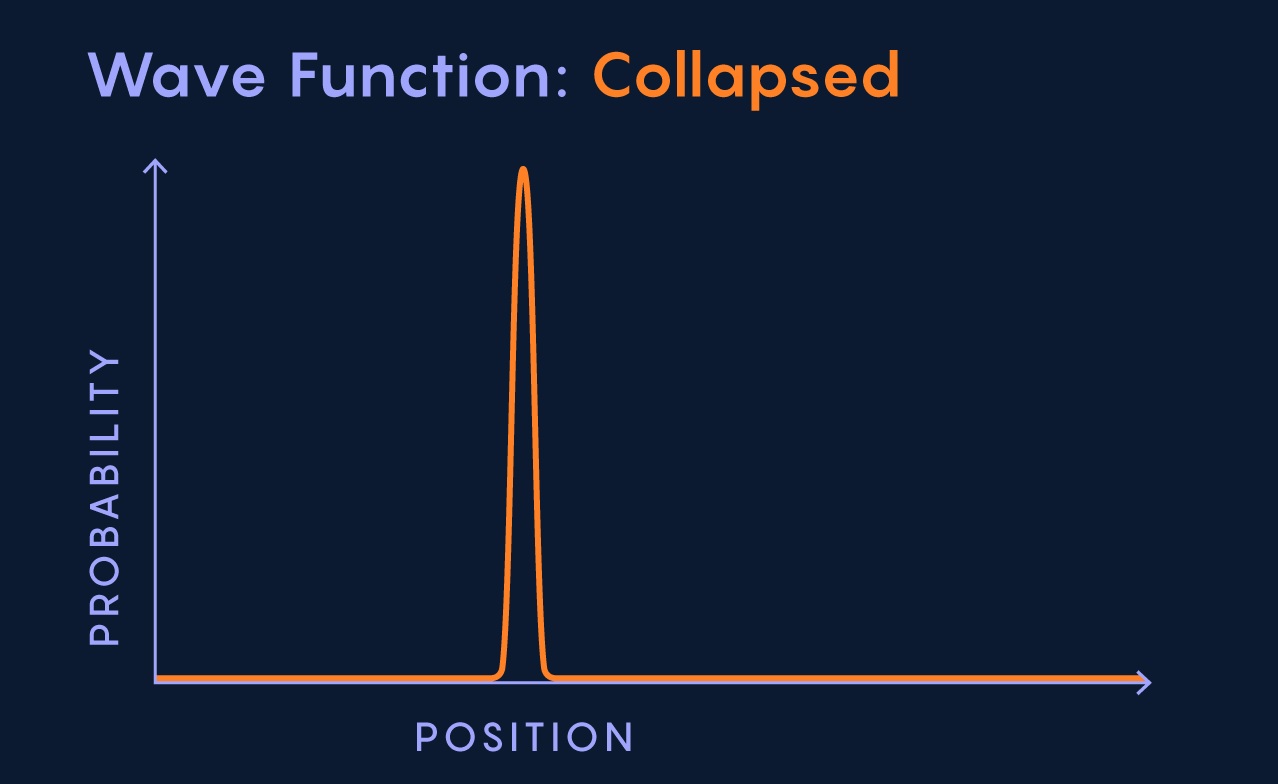
Wave Function Collapse
Wave function collapse refers to the sudden transition from a superposition of states to a single definite state when a measurement is made. This concept highlights the role of observation in determining the outcome of quantum systems.
The Uncertainty Principle
The uncertainty principle, formulated by Werner Heisenberg, states that there is a limit to how precisely we can measure certain pairs of physical properties, like position and momentum, simultaneously. The wave function encapsulates this inherent uncertainty in quantum mechanics.
Wave Function Evolution
The wave function evolves over time according to the Schrödinger equation, which describes the dynamics of quantum systems. It allows us to predict and understand the behavior of particles as they move through space and interact with their environment.
Wave Functions and Energy Levels
Wave functions are intimately connected to the concept of energy levels in quantum mechanics. They help determine the allowed energy states that particles can occupy within a given system.
Wave Function Symmetry
Wave functions exhibit different types of symmetry, such as symmetric or antisymmetric, depending on the characteristics of the particles they describe. These symmetries play a crucial role in understanding the properties and behavior of quantum systems.
Entanglement and Wave Functions
Wave functions are central to understanding the phenomenon of quantum entanglement, where the states of two or more particles become intrinsically linked. This bizarre connection persists even when the particles are widely separated.
Wave Functions and Quantum Computing
Wave functions are the foundation of quantum computing algorithms. They allow for the manipulation of qubits, the basic units of information in quantum computers, and enable powerful computational capabilities.
Wave Function Collapse and Measurement
The concept of wave function collapse is closely tied to the process of measurement in quantum mechanics. When we measure a property of a quantum system, the wave function collapses to a single state that corresponds to the measured value.
The Many-Worlds Interpretation
According to the controversial many-worlds interpretation of quantum mechanics, the wave function does not collapse but instead branches into multiple universes, each representing a different outcome of a measurement. This interpretation challenges our conventional understanding of reality.
These twelve mind-blowing facts about wave function offer just a glimpse into the intriguing world of quantum mechanics. The deeper we delve into this field, the more we realize how marvelously complex and mysterious the quantum realm truly is. The wave function serves as our mathematical tool to comprehend and navigate this extraordinary domain, where particles can exist in multiple states and their destinies are governed by probabilities.
Conclusion
In conclusion, wave function is a fundamental concept in quantum mechanics that describes the behavior of particles and waves. Through its mathematical representation, the wave function provides information about the probability of finding a particle at different points in space, as well as its energy and momentum. The wave function has many mind-blowing aspects that continue to fascinate scientists and researchers.
From its duality nature, where particles can exhibit both wave-like and particle-like properties, to its role in determining the energy levels of atoms and molecules, the wave function plays a crucial role in understanding the quantum world. Furthermore, the concept of entanglement introduces the mind-boggling idea of particles being interconnected regardless of distance, paving the way for cutting-edge technologies like quantum computing.
Exploring the intricacies of wave function opens up a world of wonder and deepens our knowledge of the fundamental nature of reality. As scientists continue to unravel the mysteries of the quantum world, we can expect even more mind-blowing facts about wave function to emerge, expanding our understanding of the universe.
FAQs
1. What is wave function in quantum mechanics?
In quantum mechanics, the wave function is a mathematical description that represents the state of a particle or a system of particles. It provides information about the probability of finding a particle at different positions and the probability distribution of its properties.
2. What is wave-particle duality?
Wave-particle duality is the concept in quantum mechanics that particles can exhibit both wave-like and particle-like properties. This means that particles can behave as waves under certain conditions and as particles with well-defined positions and momenta under other conditions.
3. What is the significance of entanglement in wave function?
Entanglement is a phenomenon in which two or more particles become correlated to the extent that the states of the particles cannot be described independently. This concept is significant in wave function as it allows for the possibility of instantaneous communication and the development of technologies like quantum computing.
4. How does wave function determine the energy levels of atoms and molecules?
The wave function of an atom or molecule contains information about the possible energy levels that the electrons can occupy. Solving the Schrödinger equation using the wave function provides a set of quantized energy levels, which determine the electronic structure and stability of atoms and molecules.
5. Can wave function be observed or measured directly?
No, the wave function itself cannot be observed or measured directly. However, the probability distribution described by the wave function can be measured experimentally, allowing scientists to infer information about the behavior and properties of particles.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.