When it comes to understanding the fascinating world of chemistry, one concept that stands out is the concept of a “half-cell.” A half-cell is a vital component in many chemical reactions and plays a crucial role in the field of electrochemistry. By harnessing the power of half-cells, scientists and researchers have been able to unlock groundbreaking discoveries and advancements.
In this article, we will delve into the realm of half-cells and explore eight mind-blowing facts about them. From their importance in understanding cell potentials to their role in fuel cells and batteries, we will uncover the hidden secrets and incredible capabilities of these microscopic powerhouses. So, buckle up and get ready to have your mind blown by the fascinating science behind half-cells!
Key Takeaways:
- Half-cells are like the building blocks of batteries and fuel cells, helping to generate electricity by reacting with each other.
- Understanding half-cell potential and reactions is like unlocking the secrets of how batteries work, from balancing equations to maintaining electrical neutrality.
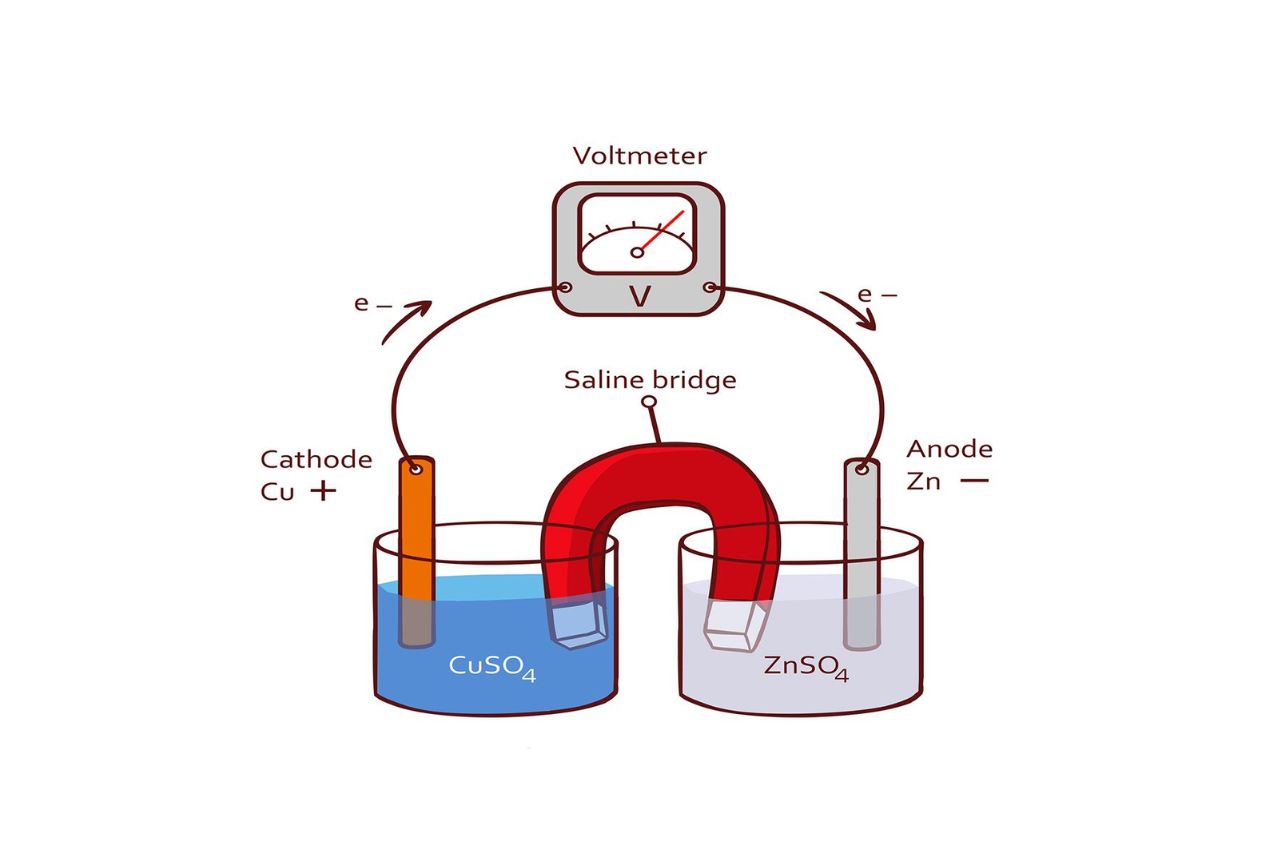
Half-Cell – The Building Block of Electrochemical Cells
The concept of a half-cell is fundamental to understanding electrochemical cells. It serves as the basic unit that comprises the anode or cathode in a cell. Whether it’s a simple battery or a complex fuel cell, the half-cell plays a crucial role in the generation of electricity.
Half-Cell Potential – A Measure of Reactivity
Each half-cell possesses a specific potential known as the half-cell potential. This measure determines the reactivity or tendency of a half-cell to lose or gain electrons. By comparing the half-cell potentials of different cells, we can predict the direction of electron flow and determine cell voltage.
Nernst Equation – Unlocking Half-Cell Potential
The Nernst equation is a powerful tool used to calculate the half-cell potential under non-standard conditions. By considering factors such as temperature, concentration, and pressure, this equation helps us understand the behavior of half-cells in different environments, enabling us to optimize cell performance.
Half-Cell Reactions – Balancing Redox Equations
Identifying and balancing half-cell reactions is essential in understanding the overall redox reactions occurring in electrochemical cells. Through these reactions, electrons are transferred, and chemical changes take place, allowing the conversion of chemical energy into electrical energy.
Salt Bridge – Maintaining Electrical Neutrality
In a complete electrochemical cell, a salt bridge is used to connect the half-cells. Its primary function is to maintain electrical neutrality by allowing the flow of ions between the two half-cells. This prevents the buildup of charges and ensures the continuity of the redox reactions.
Standard Hydrogen Electrode (SHE) – Reference for Electrode Potential
The Standard Hydrogen Electrode (SHE) serves as the reference half-cell in electrochemistry. It has a defined potential of zero volts, against which the potentials of other half-cells are measured. This standardization allows for accurate comparison and evaluation of different half-cell potentials.
Half-Cell Materials – A Wide Range of Options
Half-cell materials can vary widely depending on the application. From metals like zinc and copper to non-metals like graphite and lead dioxide, a diverse range of materials are used to create different half-cells. This versatility allows for the design and optimization of electrochemical systems for various purposes.
Half-Cell in Nature – Biological Significance
The concept of the half-cell is not limited to artificial electrochemical cells. In fact, half-cell reactions are essential for many biological processes, such as cellular respiration and photosynthesis. Understanding the principles of half-cell reactions helps us comprehend the fundamental processes of life itself.
These 8 mind-blowing facts about half-cell illustrate its significance in the field of electrochemistry and beyond. Whether you are studying the principles of electricity or exploring the wonders of biological systems, half-cells play a crucial role in our understanding of the natural and technological world.
Conclusion
In conclusion, understanding the concept of a half-cell is essential in the field of chemistry. These mind-blowing facts about half-cell shed light on its significance in various chemical reactions and electrochemical processes. From its role in battery technologies to its application in corrosion prevention, half-cells play a crucial role in modern chemistry.
By comprehending the intricacies of half-cell reactions, scientists and researchers can further advance their knowledge and develop innovative solutions for a wide range of practical applications. The study of half-cell reactions continues to unveil new possibilities in energy storage, environmental protection, and many other areas of scientific study.
As the field of chemistry progresses, it is essential to stay informed about emerging concepts like half-cell and their implications. By exploring these mind-blowing facts, we can deepen our understanding of the intricate world of chemistry and its practical applications.
FAQs
Q: What is a half-cell in chemistry?
A: A half-cell is a component of an electrochemical cell that consists of an electrode and a conductive solution. It is one of the fundamental units used to study and analyze redox reactions.
Q: How does a half-cell work?
A: A half-cell operates by allowing either an oxidation or reduction reaction to take place at the electrode. This generates a flow of electrons and creates an electrical potential difference that drives the overall electrochemical reaction.
Q: What are the different types of half-cells?
A: There are two main types of half-cells: the anode half-cell and the cathode half-cell. The anode half-cell involves the oxidation reaction, while the cathode half-cell involves the reduction reaction.
Q: What are some applications of half-cells?
A: Half-cells have various applications in chemistry and industry. They are used in batteries, fuel cells, electroplating processes, and corrosion prevention methods.
Q: Why is understanding half-cells important in chemistry?
A: Understanding half-cells is crucial because they form the basis for understanding and predicting electrochemical reactions. It allows scientists and researchers to develop new technologies and solutions in the fields of energy storage, environmental protection, and electrochemical synthesis.
Unravel more electrochemistry secrets by exploring the Nernst equation, a powerful tool for calculating half-cell potential. Dive deeper into redox reactions, the driving force behind countless chemical processes. Gain a comprehensive understanding of electrode potential and its significance in electrochemical systems.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
