Unit cells are the fundamental building blocks of crystals, playing a crucial role in understanding the structure and properties of materials. These small repeating units form the basis for the incredible diversity and complexity exhibited by crystals. In the field of chemistry, unit cells are of paramount importance, as they help us unravel the mysteries of various chemical compounds and their behavior.
In this article, we will delve into the fascinating world of unit cells and explore 19 astounding facts about them. From their discovery and classification to their role in crystallography and the impact they have on the properties of materials, we will uncover the intricate details that make unit cells such an essential concept in the realm of chemistry. So, let’s dive in and unravel the wonders of unit cells!
Key Takeaways:
- Unit cells are like the building blocks of crystals, coming in different shapes and sizes. They help scientists understand the properties of materials and have been around since ancient times!
- By studying unit cells, scientists can unlock the secrets of crystal symmetry and material properties. This knowledge is essential in fields like chemistry, physics, and engineering, shaping our understanding of the world around us.
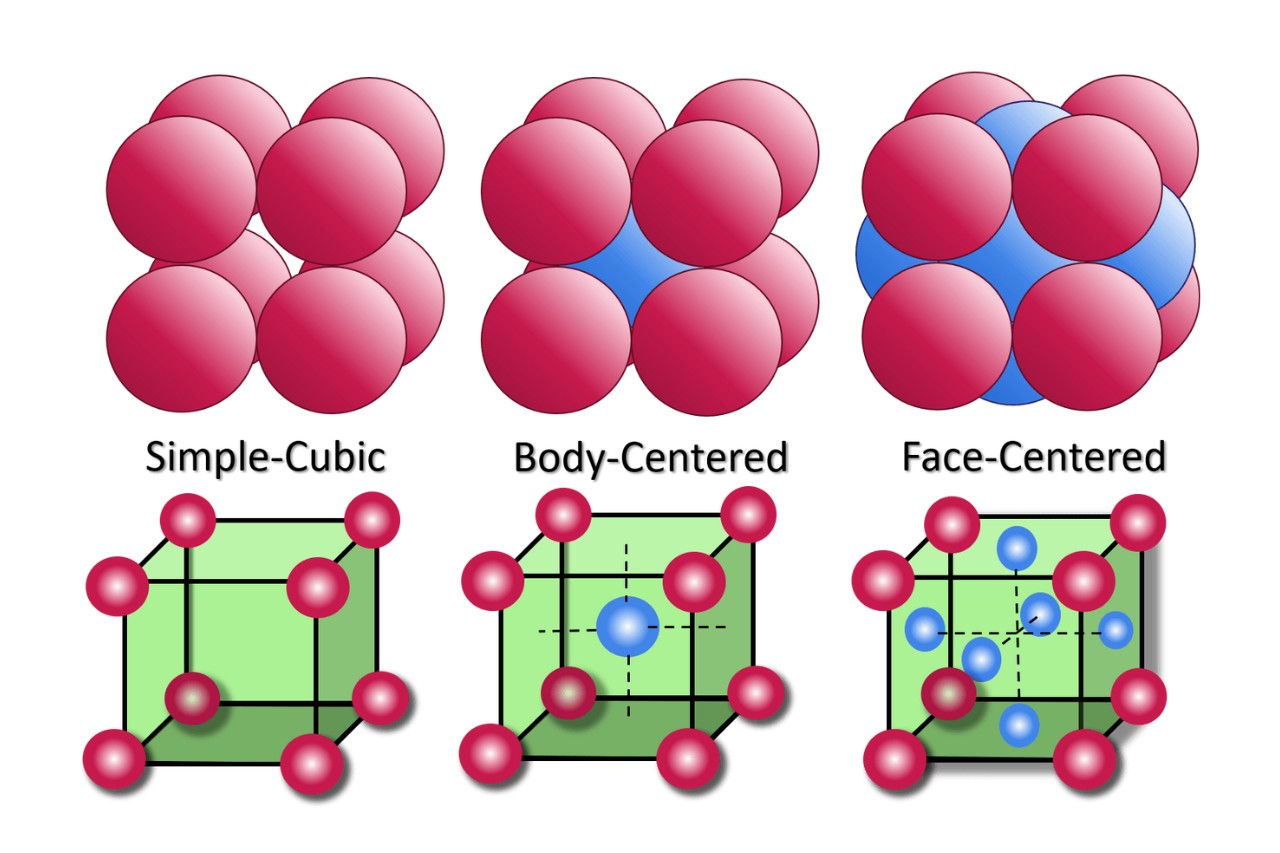
The unit cell is the basic building block of a crystal lattice.
Within the field of crystallography, the unit cell is the smallest repeating unit that makes up a crystal structure. It represents the fundamental geometric arrangement of atoms or molecules in a crystal lattice.
Unit cells can have different shapes and sizes.
While the most common unit cell shape is a cube, known as a cubic unit cell, there are also other shapes such as hexagonal, tetragonal, orthorhombic, and rhombohedral unit cells.
A unit cell is characterized by its lattice parameters.
The lattice parameters are the lengths of the edges and the angles between them that define the unit cell’s shape and size.
There are seven crystal systems based on unit cell characteristics.
The crystal systems are cubic, tetragonal, orthorhombic, rhombohedral, hexagonal, monoclinic, and triclinic.
Unit cells provide a framework for understanding crystal symmetry.
By studying the arrangement of atoms or molecules in a unit cell, scientists can determine the symmetry elements and operations present in the crystal lattice.
Unit cells can be classified as primitive or non-primitive.
A primitive unit cell contains only one lattice point, while a non-primitive unit cell contains more than one lattice point.
The unit cell concept is crucial in crystallography and materials science.
Understanding unit cells allows researchers to analyze and predict physical properties of materials, such as conductivity, optical properties, and mechanical behavior.
Unit cells can be used to determine the crystal structure of a substance.
By studying the diffraction pattern of X-rays or other radiation, scientists can deduce the arrangement of atoms or molecules in a crystal lattice using the concept of unit cells.
The arrangement of atoms in a unit cell can affect material properties.
Small changes in the arrangement of atoms within a unit cell can result in significant variations in properties such as density, electrical conductivity, and magnetic behavior.
Unit cells can exhibit translational symmetry.
Translational symmetry refers to the regular repetition of the unit cell throughout the crystal lattice, creating a pattern that extends infinitely in all directions.
The concept of unit cells dates back to ancient times.
Ancient civilizations, such as the Greeks and Egyptians, observed and utilized the repeating patterns found in crystals, laying the foundation for the modern understanding of unit cells.
Unit cells are not limited to solid materials.
Unit cells can also be used to describe the arrangement of molecules in liquids and gases, providing insights into their physical properties and behaviors.
Unit cells can have different arrangements of atoms or molecules.
The arrangement of atoms in a unit cell can be simple and regular, or it can be complex and irregular, depending on the nature of the crystal structure.
Unit cells can exhibit different types of symmetry.
Symmetry operations, such as rotations, translations, and reflections, can be present within the unit cell, giving rise to different types of crystal symmetry.
The unit cell concept is essential in the study of solid-state physics.
By understanding the unit cell, physicists can investigate phenomena such as band structure, electron behavior, and thermal properties in crystalline materials.
Unit cells can be visualized in three dimensions.
While unit cells are typically represented as two-dimensional drawings, they actually exist in three-dimensional space and can be visualized using advanced imaging techniques.
The concept of unit cells is used in various scientific disciplines.
Unit cells are not limited to crystallography but are also employed in chemistry, materials science, geology, and engineering to understand and manipulate the properties of diverse materials.
The study of unit cells continues to advance our understanding of matter.
As technology improves, new techniques and tools allow scientists to explore and unravel the mysteries of unit cells at increasingly smaller scales, opening up exciting possibilities for future discoveries and applications.
In summary, the unit cell is a fundamental concept in crystallography and materials science.
It serves as the building block for understanding crystal structures, symmetry, and the properties of various materials. The concept of unit cells has a rich historical background and continues to play a crucial role in advancing our knowledge of matter in the modern scientific era.
Conclusion
In conclusion, understanding unit cells is crucial in the study of chemistry and materials science. Unit cells are the building blocks of crystals and play a vital role in determining the properties and behavior of solid materials. Through unit cell analysis, scientists can gain valuable insights into the atomic arrangement, symmetry, and lattice parameters of crystalline structures. This knowledge helps in various fields including drug discovery, metallurgy, and nanotechnology. By exploring the 19 astounding facts about unit cells, we have highlighted the intricacies and significance of these fundamental structures. From the discovery of Bravais lattices to the exploration of crystallographic defects, unit cells continue to fascinate and influence our understanding of the world around us.
FAQs
1. What is a unit cell?
A unit cell is the smallest repeating structural unit that makes up a crystal lattice.
2. What is the significance of unit cells?
Unit cells help determine the properties and behavior of solids, such as their conductivity, melting point, and optical properties.
3. How are unit cells classified?
Unit cells are classified based on their symmetry into seven crystal systems: cubic, tetragonal, orthorhombic, monoclinic, triclinic, hexagonal, and rhombohedral.
4. What are Bravais lattices?
Bravais lattices are the 14 unique three-dimensional spatial arrangements of lattice points that can fill space.
5. What is a crystallographic defect?
A crystallographic defect is a fault or irregularity in the atomic arrangement of a crystal lattice that affects its properties.
6. How do unit cells contribute to drug discovery?
Unit cell analysis helps in determining the structure of drug molecules and their interaction with target proteins, aiding in drug design and development.
7. What role do unit cells play in metallurgy?
Unit cells help understand the structure and properties of metals, which is essential for optimizing their alloys and improving mechanical characteristics.
8. How are unit cells studied?
Unit cells are studied using techniques such as X-ray diffraction, electron microscopy, and computational modeling.
9. Can different materials have the same unit cell?
Yes, different materials can have the same unit cell if their atomic arrangements and lattice parameters match.
10. How do unit cells contribute to nanotechnology?
Unit cell analysis aids in understanding the atomic-scale structures and properties of nanomaterials, guiding their synthesis and applications in various fields.
Exploring unit cells is just the beginning of a fascinating journey into the world of crystallography. Uncover the profound impact of symmetry on the laws of physics through Noether's theorem. Dive deeper into the intricate patterns of crystal lattices and their mesmerizing structures. Investigate the intriguing nature of crystal defects and how they shape the properties of materials.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


