Arrhenius acid, named after the Swedish chemist Svante Arrhenius, is a fundamental concept in chemistry. Acids play a crucial role in numerous chemical reactions and have a significant impact on our daily lives. Understanding the properties and characteristics of Arrhenius acids is essential for a comprehensive understanding of chemistry.
In this article, we will explore 12 astounding facts about Arrhenius acid that will captivate your curiosity and deepen your understanding of this fascinating topic. From its definition and key properties to its applications and examples, we will delve into the world of Arrhenius acid and unravel its secrets. So, let’s jump in and discover some intriguing facts about Arrhenius acid!
Key Takeaways:
- Arrhenius Acid donates protons in water, found in fruits and stomach acid, and plays a vital role in industries like pharmaceuticals and food processing.
- Arrhenius Acid can be strong or weak, causes color changes in indicators, and is crucial for understanding pH and reaction rates in chemistry.
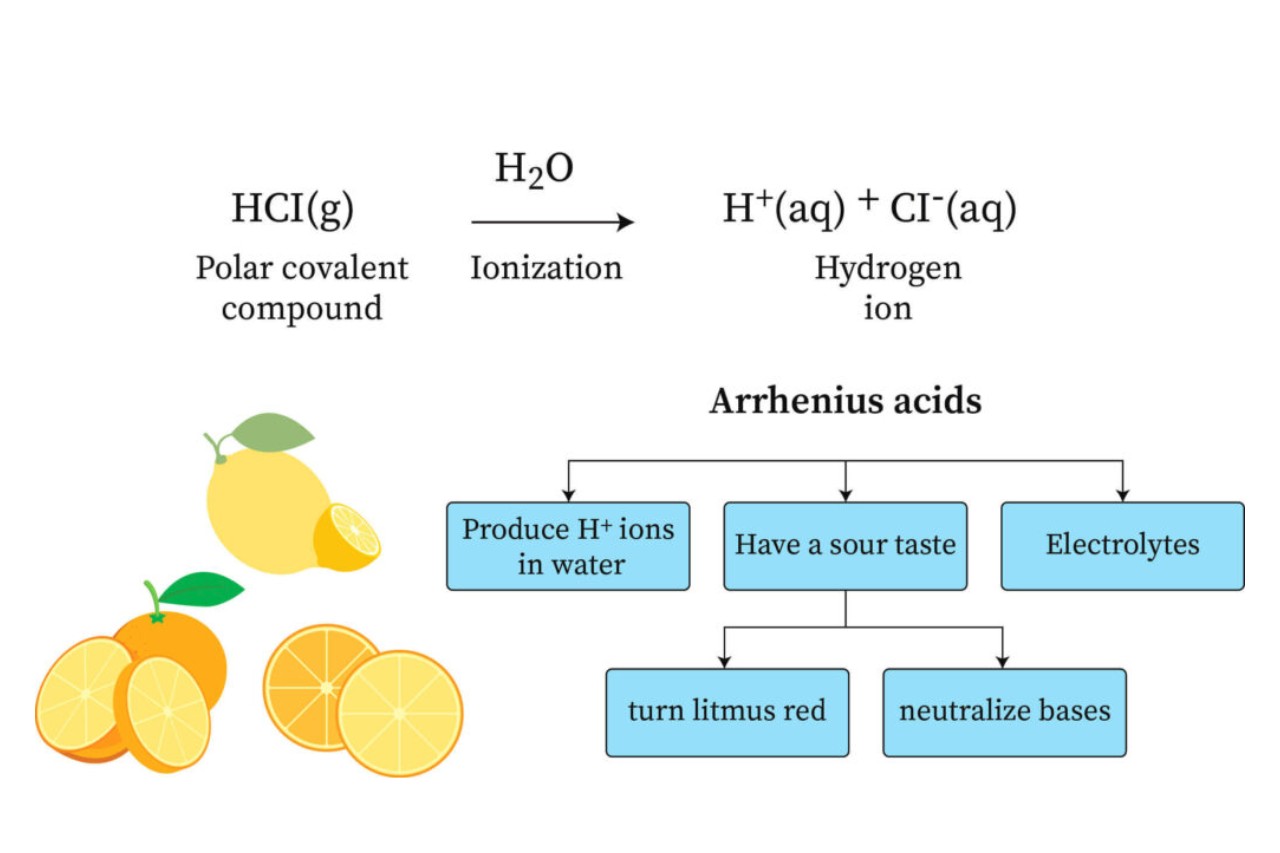
Arrhenius Acid is defined as a substance that donates protons (H+) in aqueous solutions.
Arrhenius Acid is characterized by its ability to release hydrogen ions when dissolved in water. This leads to an increase in the concentration of H+ ions, resulting in a lower pH level.
Arrhenius Acid exhibits several properties, including sour taste and the ability to react with metals, bases, and carbonates.
The sour taste of Arrhenius Acid is a result of its acidic nature. Additionally, it can react with metals, bases, and carbonates to form salts and other chemical compounds.
Arrhenius Acid can be found in various substances, such as citrus fruits, vinegar, and even stomach acid.
Common examples of Arrhenius Acid include citric acid present in citrus fruits, acetic acid found in vinegar, and hydrochloric acid present in our stomachs.
Arrhenius Acid plays a vital role in chemical reactions and is widely used in industries, including pharmaceuticals and food processing.
Due to its ability to catalyze chemical reactions, Arrhenius Acid is extensively utilized in industries such as pharmaceuticals, food processing, and manufacturing.
Arrhenius Acid can be classified into strong acids, weak acids, and superacids based on their dissociation constants.
Based on their ability to dissociate in aqueous solutions, Arrhenius Acid can be categorized into strong acids (complete dissociation), weak acids (partial dissociation), and superacids (exceptionally strong acids).
The concept of Arrhenius Acid was introduced by Svante Arrhenius, a Swedish chemist, in the late 19th century.
Svante Arrhenius, a prominent Swedish chemist, proposed the concept of Arrhenius Acid in 1884, revolutionizing the understanding of acidic compounds.
Arrhenius Acid can cause skin and eye irritation, and proper safety precautions should be taken while handling concentrated acids.
Concentrated Arrhenius Acids can be corrosive and harmful. It is important to handle them with utmost care, taking necessary safety measures to prevent skin and eye irritation.
Arrhenius Acid can alter the color of certain indicators, leading to the formation of characteristic color changes.
The reaction of Arrhenius Acids with specific indicators can result in distinct color changes, which are often used to detect the presence of acids.
Arrhenius Acid can react with Arrhenius Bases to form salts through a process known as neutralization.
When Arrhenius Acid reacts with Arrhenius Bases, they undergo a neutralization reaction, producing salts and water as byproducts.
The strength of Arrhenius Acid is determined by its tendency to donate protons and its equilibrium constant.
The strength of an Arrhenius Acid is determined by its ability to donate protons and its equilibrium constant, which is a measure of the tendency for the acid to dissociate.
Arrhenius Acid is an essential component in the study of pH and the acid-base balance.
Understanding Arrhenius Acid is crucial in the study of pH and maintaining the acid-base balance in various biological and chemical systems.
Arrhenius Acid is an integral part of the Arrhenius Equation, which relates reaction rates to temperature.
In the field of chemical kinetics, the Arrhenius Equation incorporates Arrhenius Acid as a fundamental variable in the calculation of reaction rates as a function of temperature.
These 12 astounding facts about Arrhenius Acid highlight its significance in chemistry and its widespread applications in various domains. From its ability to donate protons to its role in industrial processes, Arrhenius Acid continues to captivate scientists and researchers worldwide.
Conclusion
In conclusion, Arrhenius acids are a fascinating aspect of chemistry that have revolutionized our understanding of chemical reactions and their behavior in aqueous solutions. These acids, named after the Swedish chemist Svante Arrhenius, have played a crucial role in various industries and scientific research.From understanding common household acids to exploring the complexities of acid-base reactions, Arrhenius acids have provided a framework for comprehension and analysis. Their unique properties, such as producing hydrogen ions (H+) when dissolved in water, make them an essential component of many chemical processes.Furthermore, the groundbreaking work of Svante Arrhenius and his acid dissociation theory has paved the way for advancements in numerous fields, including medicine, environmental science, and materials science. The study of Arrhenius acids continues to be valuable in practical applications and theoretical explorations.In summary, the 12 astounding facts about Arrhenius acids showcased their significance in the realm of chemistry. As we delve deeper into the study of acids and their role in chemical reactions, the influence of Arrhenius acids remains substantial and continues to shape our understanding of the fascinating world of chemical interactions.
FAQs
1. What are Arrhenius acids?
Arrhenius acids are substances that, when dissolved in water, produce hydrogen ions (H+). They are named after the Swedish chemist Svante Arrhenius, who proposed the acid dissociation theory.
2. How do Arrhenius acids behave in aqueous solutions?
Arrhenius acids donate hydrogen ions (H+) when dissolved in water. These hydrogen ions then interact with water molecules to form hydronium ions (H3O+).
3. Can you provide examples of Arrhenius acids?
Some examples of Arrhenius acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH).
4. How are Arrhenius acids different from other types of acids?
Arrhenius acids specifically refer to substances that produce hydrogen ions (H+) when dissolved in water. Other types of acids, such as Lewis acids or Brønsted-Lowry acids, have different definitions and characteristics.
5. What are the applications of Arrhenius acids?
Arrhenius acids are used in various industries, including chemical manufacturing, pharmaceuticals, and environmental analysis. They also play a crucial role in acid-base reactions and are studied extensively in chemistry and related fields.
6. Are Arrhenius acids always corrosive or dangerous?
While some Arrhenius acids can be corrosive or dangerous, not all of them are. The level of acidity and potential harm depends on the specific acid and its concentration.
7. Can Arrhenius acids be found naturally?
Yes, many Arrhenius acids can be found naturally in various substances, such as citrus fruits, vinegar, and even our own stomach acids.
8. How did Svante Arrhenius contribute to our understanding of Arrhenius acids?
Svante Arrhenius proposed the acid dissociation theory, which laid the foundation for our understanding of Arrhenius acids. His work greatly contributed to the field of chemistry.
9. Are there any limitations to the Arrhenius acid concept?
While the concept of Arrhenius acids is widely accepted, it has its limitations. It does not account for acids that do not produce hydrogen ions (H+) when dissolved in water, such as Lewis acids or Brønsted-Lowry acids.
10. Can Arrhenius acids be neutralized?
Yes, Arrhenius acids can be neutralized by reacting them with bases. The resulting reaction produces water and a salt.
11. Are all common acids considered Arrhenius acids?
No, not all common acids are Arrhenius acids. Some common acids, such as carbonic acid (H2CO3), are not classified as Arrhenius acids because they do not fully dissociate in water.
12. Can Arrhenius acids exist in forms other than aqueous solutions?
Arrhenius acids can exist in forms other than aqueous solutions. They can be in the gas phase, as in the case of hydrochloric acid gas (HCl), or in solid form, such as citric acid crystals.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
