The Acid Dissociation Constant (Ka) is a fundamental concept in the field of chemistry that plays a crucial role in understanding the behavior of acids in aqueous solutions. It quantifies the extent to which an acid dissociates into its ions in water and provides important information about the strength of the acid. While the concept of Ka may seem straightforward, it holds a few surprising facts that are worth exploring.
In this article, we will delve into 9 surprising facts about the Acid Dissociation Constant (Ka) that will deepen your understanding of this essential chemical phenomenon. From the influence of temperature on Ka values to the connection between Ka and acid strength, we will unravel the lesser-known aspects of Ka that often go unnoticed.
So, buckle up and get ready to uncover these intriguing facts about the Acid Dissociation Constant (Ka) that will enhance your knowledge of the fascinating world of chemistry.
Key Takeaways:
- Ka measures acid strength and can vary for different acids. Lower pKa values indicate stronger acids. Temperature and solvent properties can also affect Ka values, influencing pH calculations and acid-base titrations.
- Conjugate bases have their own dissociation constants (Kb). The ratio of [A-] to [HA] affects Ka value, determining acid strength. Ka values play a crucial role in acid-base titrations for concentration determination.
Acid Dissociation Constant (Ka) measures the strength of an acid.
Acid Dissociation Constant (Ka) is a crucial concept in chemistry that quantifies the strength of an acid. It represents the equilibrium constant for the dissociation of the acid in water, indicating the extent to which the acid releases its hydrogen ions. By understanding the value of Ka, scientists can predict the behavior and reactivity of acids in various chemical reactions.
Ka values can vary across different acid molecules.
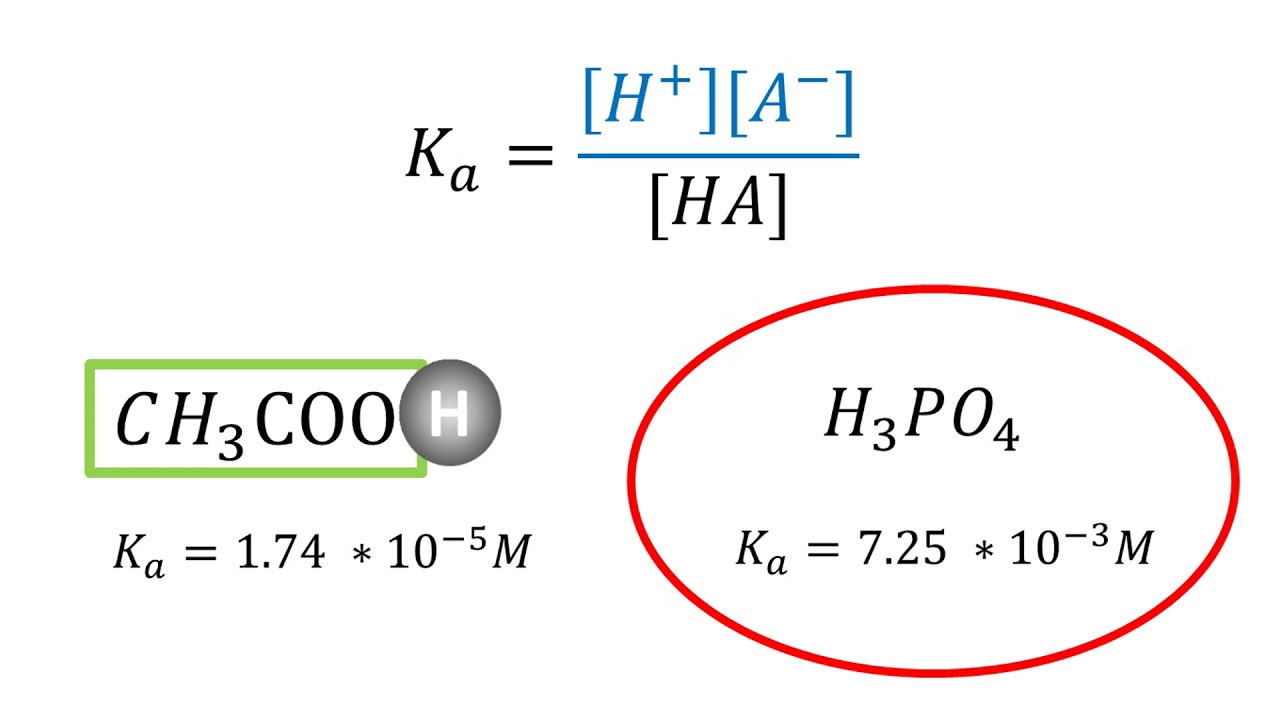
Each acid has its own unique Ka value, which depends on its chemical structure and characteristics. Strong acids, such as hydrochloric acid (HCl), have high Ka values, indicating complete dissociation in water. On the other hand, weak acids like acetic acid (CH3COOH) have lower Ka values, indicating only partial dissociation. The variation in Ka values allows chemists to compare the acid strength and determine the relative acidity of different substances.
The pKa value is the negative logarithm of Ka.
To simplify the representation of Ka values, chemists use the pKa scale. The pKa value is obtained by taking the negative logarithm (base 10) of the Ka value. It provides a more convenient and manageable scale for comparing acid strengths, as lower pKa values correspond to stronger acids. Thus, chemists often use pKa values to analyze and predict the behavior of acids in reactions.
Ka values can be affected by temperature.
The value of Ka is temperature-dependent, meaning that it can change as the temperature of the system varies. In general, higher temperatures tend to increase the Ka value for most acid dissociations. This is because higher temperatures provide more energy for molecular collisions, leading to more successful dissociation of the acid molecules. However, the specific temperature dependence may vary for different acid systems.
Ka values can be influenced by solvent properties.
The solvent in which an acid is dissolved can also affect its Ka value. Water is the most common solvent used in acid dissociation studies, but other solvents can significantly impact the dissociation process. Solvents with higher dielectric constants tend to stabilize the ions produced by acid dissociation, resulting in higher Ka values. Solvents with low dielectric constants, such as nonpolar solvents, can hinder the dissociation and lead to lower Ka values.
Ka values can be used to calculate pH.
pH is a measure of the acidity or alkalinity of a solution and is closely related to the Ka value. By using the Ka value of an acid and its concentration, it is possible to calculate the pH of a solution using the appropriate mathematical equations. This allows chemists to quantitatively determine the acidity of a solution based on its Ka value and concentration.
Conjugate bases have their own dissociation constants, Kb.
When an acid donates a hydrogen ion, it forms a conjugate base. These conjugate bases can also undergo dissociation and have their own equilibrium constants called Kb values. Kb represents the dissociation constant of the base and indicates the strength of the base. Just like Ka, Kb values can vary depending on the chemical structure and properties of the base molecule.
The ratio of [A-] to [HA] affects Ka value.
The Ka value of an acid is calculated based on the ratio of the concentration of the dissociated form of the acid ([A-]) to the undissociated form ([HA]). At equilibrium, the Ka value is equal to [A-] multiplied by [H+] concentration, divided by [HA] concentration. The higher the ratio of [A-] to [HA], the larger the Ka value, indicating a stronger acid. Conversely, a smaller ratio results in a lower Ka value, representing a weaker acid.
Ka values play a key role in acid-base titrations.
Acid-base titration is a common technique used in analytical chemistry to determine the concentration of an acid or a base in a solution. Ka values are crucial in titration calculations, as they help determine the equivalence point and understand the reaction between the acid and base. By utilizing the Ka value, scientists can accurately measure the concentration of an unknown acid or base in a solution.
Conclusion
In conclusion, the acid dissociation constant (Ka) is a fundamental concept in Chemistry that plays a crucial role in understanding the behavior of acids in solution. We have explored some surprising facts about Ka, shedding light on its significance in various chemical reactions and equilibria.We have learned that Ka measures the extent to which an acid dissociates into its ions in solution, with higher Ka values indicating stronger acids. Additionally, we discovered that Ka values can be influenced by factors such as temperature, solvent, and the presence of other ions.Furthermore, the knowledge of Ka can be used to calculate the pH of a solution, determine the strength of an acid, or predict the direction of a chemical reaction. Its applications extend to fields like pharmaceuticals, environmental sciences, and industrial chemistry.Understanding the nuances of the acid dissociation constant (Ka) empowers chemists to make informed decisions, design experiments, and develop innovative solutions. It deepens our understanding of acid-base chemistry and paves the way for further advancements in the field.
FAQs
Q: What is the acid dissociation constant (Ka)?
A: The acid dissociation constant, Ka, is a measure of the extent to which an acid dissociates into its ions in solution. It quantifies the strength of an acid.
Q: How is the acid dissociation constant (Ka) calculated?
A: The formula to calculate Ka is: Ka = [H+][A-] / [HA], where [H+] is the concentration of hydrogen ions, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the acid.
Q: What does a higher Ka value indicate?
A: A higher Ka value indicates a stronger acid. It means that the acid has a greater tendency to dissociate into its ions in solution.
Q: How does temperature affect the acid dissociation constant (Ka)?
A: Generally, increasing the temperature increases the value of Ka for an acid. Higher temperatures provide more energy for the acid molecules to dissociate into ions.
Q: Can the acid dissociation constant (Ka) be influenced by the solvent?
A: Yes, the choice of solvent can impact the value of Ka. Different solvents can have varying effects on the extent of acid dissociation.
Q: What role does the acid dissociation constant (Ka) play in pH calculations?
A: The value of Ka is used to calculate the pH of a solution. It is an essential component in the equations that relate the concentration of hydrogen ions to the acidity of a solution.
Q: Are there any practical applications of the acid dissociation constant (Ka)?
A: Yes, Ka is widely used in various fields. It is instrumental in pharmaceutical research, environmental monitoring, and industrial chemistry, among others.
Q: Can the acid dissociation constant (Ka) be used to predict the direction of a chemical reaction?
A: Yes, the value of Ka can help determine the relative strength of reactants and products in a chemical reaction. It provides insights into the equilibrium position of the reaction.
Q: How does the knowledge of the acid dissociation constant (Ka) contribute to the field of chemistry?
A: Understanding Ka enhances our understanding of acid-base chemistry, allowing for precise measurements, accurate experimental design, and informed decision-making in chemical research and industrial applications.
Acid dissociation constants play a crucial role in chemical reactions, but there's so much more to explore in the fascinating world of chemistry. Dive into the captivating realm of chemical equilibrium, where reactions reach a delicate balance. Uncover the enigmatic properties of buffer solutions, which maintain stable pH levels in the face of change. And for a thrilling journey beyond the lab, discover the gripping facts behind the movie Split, a tale of dissociation that will keep you on the edge of your seat.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
