The Henderson-Hasselbalch Equation is a fundamental concept in the field of Chemistry that allows us to understand the relationship between the pH (acidity) and the pKa (acid dissociation constant) of a solution. Developed by the biochemist Lawrence Joseph Henderson and the Danish physiologist Karl Albert Hasselbalch, this equation has revolutionized our understanding of acid-base equilibrium.
In this article, we will explore 17 mind-blowing facts about the Henderson-Hasselbalch Equation that will deepen your understanding of this vital topic. From its historical background to its practical applications, we will dive into the fascinating world of this equation and uncover the secrets behind its remarkable significance in the study of Chemistry.
Key Takeaways:
- The Henderson-Hasselbalch equation helps scientists predict pH changes and is used in fields like medicine and drug development. It’s like a pH crystal ball for chemistry!
- Named after its creators, this equation is a pH powerhouse used in biology, medicine, and more. It’s like the superhero of acid-base equilibrium!
The Henderson-Hasselbalch equation is named after its creators
The equation was devised by a British biochemist, Lawrence Joseph Henderson, and a Swedish biochemist, Karl Albert Hasselbalch. Their collaboration led to the development of this equation, which revolutionized the way chemists analyze acid-base equilibria.
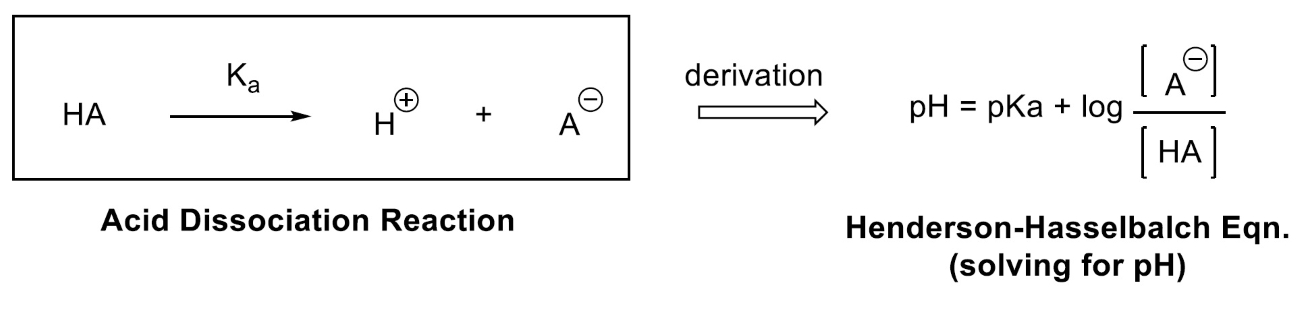
It relates the pH of a solution to the pKa and the concentrations of acid and conjugate base
The Henderson-Hasselbalch equation is expressed as pH = pKa + log([A-]/[HA]), where pH is the measure of the acidity or basicity of a solution, pKa is the negative logarithm of the acid dissociation constant, [A-] represents the concentration of the conjugate base, and [HA] represents the concentration of the acid.
The Henderson-Hasselbalch equation is widely used in biological and pharmaceutical research
Due to its applicability in studying physiological systems, the Henderson-Hasselbalch equation is extensively utilized in fields such as biochemistry, pharmacology, and medicine. It helps researchers understand and manipulate the pH conditions in various biological processes and drug formulations.
The equation allows for the prediction of pH changes upon the addition of acid or base
By manipulating the variables in the Henderson-Hasselbalch equation, scientists can predict the impact of adding acid or base to a solution. This makes it an invaluable tool for determining the pH of buffers and designing experiments that require specific pH conditions.
The Henderson-Hasselbalch equation is derived from the equilibrium constant expression
The equation is derived from the equation for the equilibrium constant of a weak acid dissociation reaction. By rearranging the terms and taking the logarithm, the Henderson-Hasselbalch equation is obtained, allowing for easy pH calculations.
It is applicable to both weak acids and weak bases
The Henderson-Hasselbalch equation is not limited to just weak acids. It can also be applied to weak bases by considering the dissociation of the conjugate acid. This flexibility makes it a versatile tool in various chemical and biological systems.
The Henderson-Hasselbalch equation is based on the assumption of ideal solutions
Like many equations in chemistry, the Henderson-Hasselbalch equation assumes ideal conditions, such as infinitely dilute solutions and complete dissociation of the acid and its conjugate base. While it may not hold true in all scenarios, it still provides valuable insights into acid-base equilibrium.
It is commonly used in the preparation of pharmaceutical formulations
Pharmaceutical scientists frequently use the Henderson-Hasselbalch equation to prepare drug formulations with specific pH values. By calculating the ratio of acid to conjugate base, they can control the acidity of the formulation, ultimately affecting drug solubility and stability.
The Henderson-Hasselbalch equation contributes to our understanding of acid-base balance in the human body
Understanding acid-base balance is crucial for maintaining optimal physiological function in the human body. The Henderson-Hasselbalch equation helps in comprehending the intricate processes involved in regulating pH and acid-base equilibrium in various biological systems.
It can be used to calculate buffering capacity
The Henderson-Hasselbalch equation plays a vital role in determining the buffering capacity of a solution. By analyzing the ratio of the concentrations of the acidic and basic components of a buffer, scientists can evaluate its ability to resist changes in pH when an acid or base is added.
The Henderson-Hasselbalch equation can be used to analyze acid-base titrations
During acid-base titrations, the Henderson-Hasselbalch equation is employed to calculate the pH at different points of the reaction. This information helps in determining the equivalence point and allows for a more precise analysis of the titration curve.
The Henderson-Hasselbalch equation is a logarithmic expression
The logarithmic nature of the Henderson-Hasselbalch equation highlights the significance of pH changes. Small changes in concentration ratios can result in significant variations in pH, making the equation a powerful tool for understanding acid-base systems.
It is taught in introductory chemistry courses
The Henderson-Hasselbalch equation is a fundamental concept taught to chemistry students at the introductory level. It forms the basis for understanding acid-base equilibrium and lays the groundwork for advanced topics such as buffer systems and biochemistry.
The Henderson-Hasselbalch equation can be used to analyze blood pH
Since blood pH is crucial for maintaining physiological functions, the Henderson-Hasselbalch equation is employed to study acid-base balance in the human body. It helps diagnose disorders such as acidosis or alkalosis and guides medical interventions to restore normal blood pH.
It is an essential tool in pharmaceutical research and development
Pharmaceutical scientists rely on the Henderson-Hasselbalch equation during drug discovery and development. It assists in optimizing drug formulations, determining drug solubility, and predicting drug behavior under different pH conditions.
The Henderson-Hasselbalch equation is applicable to various fields of science
Besides chemistry, the Henderson-Hasselbalch equation finds applications in fields such as biology, biochemistry, medicine, and environmental science. Its versatility and practical value make it a cornerstone equation in multiple scientific disciplines.
The Henderson-Hasselbalch equation is a valuable asset for understanding acid-base equilibrium
The Henderson-Hasselbalch equation allows scientists to delve into the complex world of acid-base equilibrium and pH calculations. Its straightforward formulation and broad range of applications make it an indispensable tool for chemists and researchers alike.
In conclusion, the Henderson-Hasselbalch equation is a powerful formula that underpins our understanding of acid-base equilibrium. Its incredible versatility, practicality, and widespread use in various scientific disciplines make it truly an extraordinary concept in the world of chemistry.
Conclusion
In conclusion, the Henderson-Hasselbalch equation is a fundamental concept in chemistry that plays a crucial role in understanding acid-base equilibrium. This equation allows us to calculate the pH of a solution and determine its acidity or alkalinity based on the concentrations of acid and conjugate base.The 17 unbelievable facts about the Henderson-Hasselbalch equation have shed light on its significance and practical applications. We’ve learned that this equation is not limited to just one specific area of chemistry but has implications in various fields such as biochemistry, pharmaceutical sciences, and environmental chemistry.Understanding the Henderson-Hasselbalch equation empowers scientists and researchers to make accurate predictions and calculations in a wide range of scenarios. Whether it’s determining the optimal pH for enzymatic reactions, evaluating drug solubility, or studying acid-base balance in our bodies, this equation remains a cornerstone in chemical equilibrium calculations.The Henderson-Hasselbalch equation is indeed a fascinating and powerful tool that continues to contribute to our understanding of the chemical world around us.
FAQs
Q: What is the Henderson-Hasselbalch equation?
A: The Henderson-Hasselbalch equation is an equation used in chemistry to calculate the pH of a solution based on the concentrations of acid and conjugate base.
Q: What are the key components of the Henderson-Hasselbalch equation?
A: The key components of the Henderson-Hasselbalch equation are the acidic dissociation constant (pKa value), the concentration of acid, and the concentration of its conjugate base.
Q: How is the Henderson-Hasselbalch equation used in biochemistry?
A: The Henderson-Hasselbalch equation is commonly used in biochemistry to calculate the pH of biological systems and to study the effect of pH on enzyme activity and protein folding.
Q: Can the Henderson-Hasselbalch equation be used for weak bases?
A: Yes, the Henderson-Hasselbalch equation can be used for weak bases by considering the base dissociation constant (pKb value) instead of the acid dissociation constant.
Q: How accurate is the Henderson-Hasselbalch equation?
A: The Henderson-Hasselbalch equation provides a good approximation for pH calculations in dilute solutions. However, it may not accurately predict the pH in strong acid or base solutions or in concentrated solutions.
Q: How does the Henderson-Hasselbalch equation relate to acid-base balance in the human body?
A: The Henderson-Hasselbalch equation is used to understand and maintain acid-base balance in the human body. It helps in determining the pH of body fluids, such as blood, and plays a crucial role in maintaining physiological functions.
Unraveling the mysteries of the Henderson-Hasselbalch equation is just the beginning. Dive deeper into the fascinating world of science with our captivating articles on analytical chemistry, biochemistry, and titration. From groundbreaking discoveries to mind-blowing facts, our articles will leave you craving more. Don't miss out on this opportunity to expand your knowledge and explore the incredible realm of scientific wonders. Embark on a journey of discovery today!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
