Wave-particle duality is one of the most intriguing concepts in the field of quantum mechanics. It reveals the mind-boggling idea that particles, such as electrons and photons, can exhibit characteristics of both waves and particles simultaneously. This phenomenon challenges our conventional view of the universe and has paved the way for countless scientific discoveries. In this article, we will delve into the fascinating world of wave-particle duality and explore 12 captivating facts that will leave you in awe of the quantum realm. From the mysterious properties of light to the elusive behavior of subatomic particles, get ready to be dazzled by the wonders of wave-particle duality. So, buckle up and let’s embark on this mind-expanding journey into the extraordinary realm of quantum physics!
Key Takeaways:
- Wave-particle duality means that tiny particles like electrons can act like both waves and particles. This strange behavior challenges what we thought we knew about the world of physics!
- Scientists have used wave-particle duality to create amazing things like electron microscopes and quantum computers. This concept opens up a whole new world of possibilities for understanding the universe.
The concept was first proposed by Louis de Broglie in 1924.
In his doctoral thesis, de Broglie hypothesized that particles could possess wave-like properties and proposed the wavelength associated with a particle’s momentum.
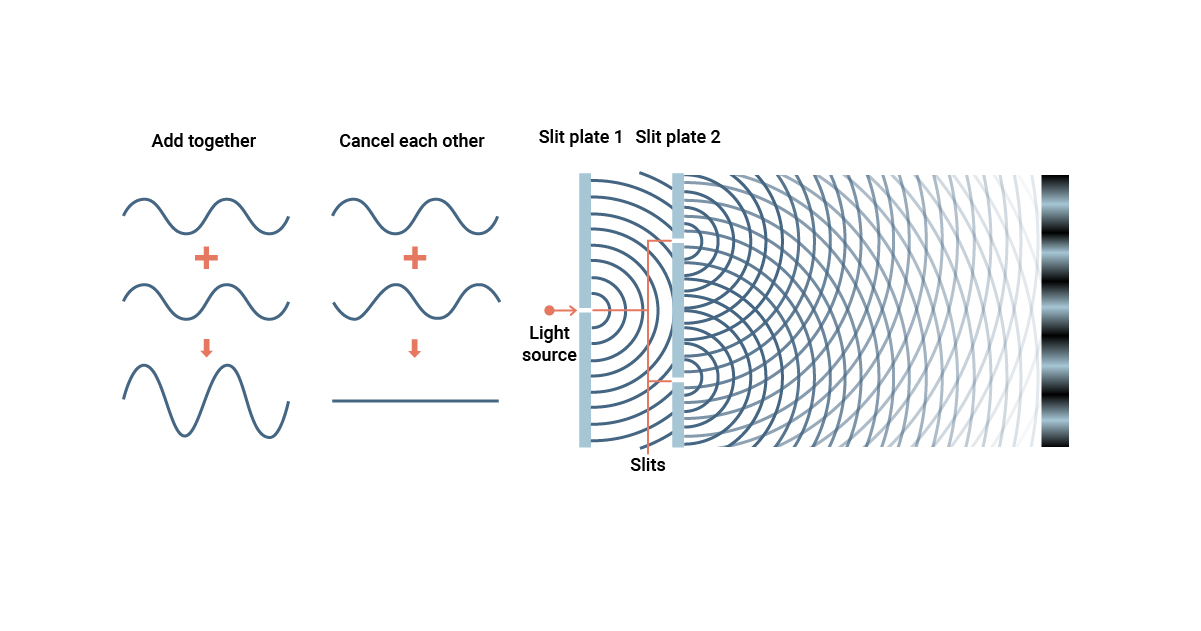
The famous double-slit experiment illustrates wave-particle duality.
In this experiment, when a beam of light or a stream of particles passes through two slits, it produces an interference pattern on the screen behind, indicating wave-like behavior.
Wave-particle duality applies to all elementary particles.
This includes electrons, protons, neutrons, and even larger particles like atoms and molecules.
Quantum mechanics provides a mathematical framework for understanding wave-particle duality.
Wave functions and probability amplitudes are used to describe the behavior of particles in quantum mechanics.
The wave function describes the probability distribution of finding a particle.
It represents the amplitude of the particle’s wave-like behavior at different locations.
The uncertainty principle is closely related to wave-particle duality.
According to the uncertainty principle, it is impossible to simultaneously know the exact position and momentum of a particle with absolute certainty.
Wave-particle duality can be observed in the phenomenon of diffraction.
Diffraction occurs when a wave encounters an obstacle or a slit and bends around it, creating a pattern of alternating light and dark regions.
The photoelectric effect provided strong evidence for the particle nature of light.
When light shines on a metal surface, it can cause the emission of electrons, which can only be explained by treating light as particles (photons).
The wave-particle duality of electrons is exploited in electron microscopy.
Electron microscopes use the wave-like nature of electrons to achieve high-resolution imaging of objects with nanoscale details.
The wave-particle duality concept challenges classical Newtonian physics.
Classical mechanics fails to accurately describe the behavior of particles at the atomic and subatomic scales where wave-particle duality becomes significant.
Researchers have conducted experiments with large molecules to observe wave-particle duality.
Larger molecules, such as carbon-60 (buckminsterfullerene), have been shown to exhibit interference patterns similar to those observed in the double-slit experiment.
Wave-particle duality has practical applications in various fields.
It is utilized in fields like quantum computing, particle physics, and the development of advanced imaging techniques.
Wave-particle duality is a fascinating concept that continues to intrigue and challenge physicists. Embracing both the wave-like and particle-like nature of matter opens up new possibilities for understanding the fundamental nature of the universe.
Conclusion
In conclusion, wave-particle duality is a fascinating concept that lies at the heart of quantum mechanics. The idea that particles can exhibit both wave-like and particle-like characteristics challenges our classical understanding of physics. With its origins dating back to the early 20th century, wave-particle duality has been the subject of extensive research and experimentation, shaping our understanding of the fundamental nature of matter and energy.By studying wave-particle duality, scientists have uncovered a myriad of captivating facts. From the groundbreaking double-slit experiment to the wave-particle complementarity principle, these discoveries have revolutionized our understanding of the quantum world. The ability of particles such as electrons, protons, and even larger entities like molecules to exhibit wave-like behavior showcases the intricate and puzzling nature of the microscopic realm.Understanding wave-particle duality is not only crucial for physicists but also opens doors to advancements in various fields. Its implications for technology, including the development of quantum computing and nanotechnology, are limitless. The study of this phenomenon continues to shed light on the fundamental nature of reality and the mysteries of the quantum world.In conclusion, wave-particle duality is a captivating and mind-bending concept that challenges our classical understanding of the universe, paving the way for remarkable scientific breakthroughs and technological advancements.
FAQs
1. What is wave-particle duality?
Wave-particle duality is the concept in quantum mechanics that suggests that particles, such as electrons and photons, can exhibit both wave-like and particle-like properties.
2. Who first proposed the idea of wave-particle duality?
The idea of wave-particle duality was first introduced by Albert Einstein in 1905 through his explanation of the photoelectric effect.
3. How does the double-slit experiment demonstrate wave-particle duality?
In the double-slit experiment, when particles are fired through two slits onto a screen, they create an interference pattern similar to that of waves, suggesting their wave-like nature. However, when observed or measured, the particles behave as discrete particles, a demonstration of their particle-like behavior.
4. What is the wave-particle complementarity principle?
The wave-particle complementarity principle states that the behavior of particles can be described either in terms of waves or particles, but not both simultaneously. The mode of observation determines whether particles exhibit wave-like or particle-like behavior.
5. Are all particles subject to wave-particle duality?
Yes, all particles, regardless of their size or mass, exhibit wave-particle duality. This includes subatomic particles like electrons and photons, as well as larger entities like molecules.
6. What are the practical applications of wave-particle duality?
Wave-particle duality has significant implications for technology, such as the development of quantum computing, nanotechnology, and improved imaging techniques.
7. Does wave-particle duality violate the laws of classical physics?
Wave-particle duality challenges the classical principles of physics, as it suggests that particles do not possess solely one nature but can exhibit both wave-like and particle-like behaviors in certain circumstances.
8. Can wave-particle duality be understood using classical physics?
No, the phenomenon of wave-particle duality cannot be explained using classical physics. It requires the framework of quantum mechanics to understand the behavior of particles at the microscopic level.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
