When it comes to understanding the properties and behavior of atoms, effective atomic radius plays a crucial role. The effective atomic radius refers to the size of an atom, taking into account the influence of its electron cloud. It is a concept that provides us with valuable insights into various phenomena in chemistry, such as bonding, reactivity, and determining the physical properties of elements.
In this article, we will delve into 12 intriguing facts about effective atomic radius that will broaden your understanding of this fundamental concept in chemistry. From the factors affecting atomic radius to its significance in predicting trends in the periodic table, we will explore the intricacies of atoms and how their sizes can vary depending on various factors.
So, get ready to embark on a journey through the world of effective atomic radius and discover fascinating facts that will deepen your appreciation for the intricacies of the atomic realm.
Key Takeaways:
- 1. Atoms have different sizes, which affect how they behave in chemical reactions. The bigger the atom, the more likely it is to form positive ions, while smaller atoms are more likely to form negative ions.
- 2. Scientists use the concept of effective atomic radius to understand how atoms interact and predict their behavior. It also helps them figure out the physical properties of different materials.
The concept of effective atomic radius is used to describe the size of an atom.
The effective atomic radius refers to the distance from the nucleus to the outermost energy level where electrons are found. It helps us understand the size of an atom and how it interacts with other atoms in chemical reactions.
Effective atomic radius can vary depending on the element and its electron configuration.
Atoms can have different electron arrangements, which affects their effective atomic radius. Elements with more electrons in the outer energy level tend to have larger effective atomic radii compared to elements with fewer outer electrons.
Effective atomic radius affects the chemical properties of an element.
The size of an atom plays a crucial role in determining its chemical behavior. Elements with larger effective atomic radii are more likely to form positive ions, while elements with smaller effective atomic radii are more likely to form negative ions.
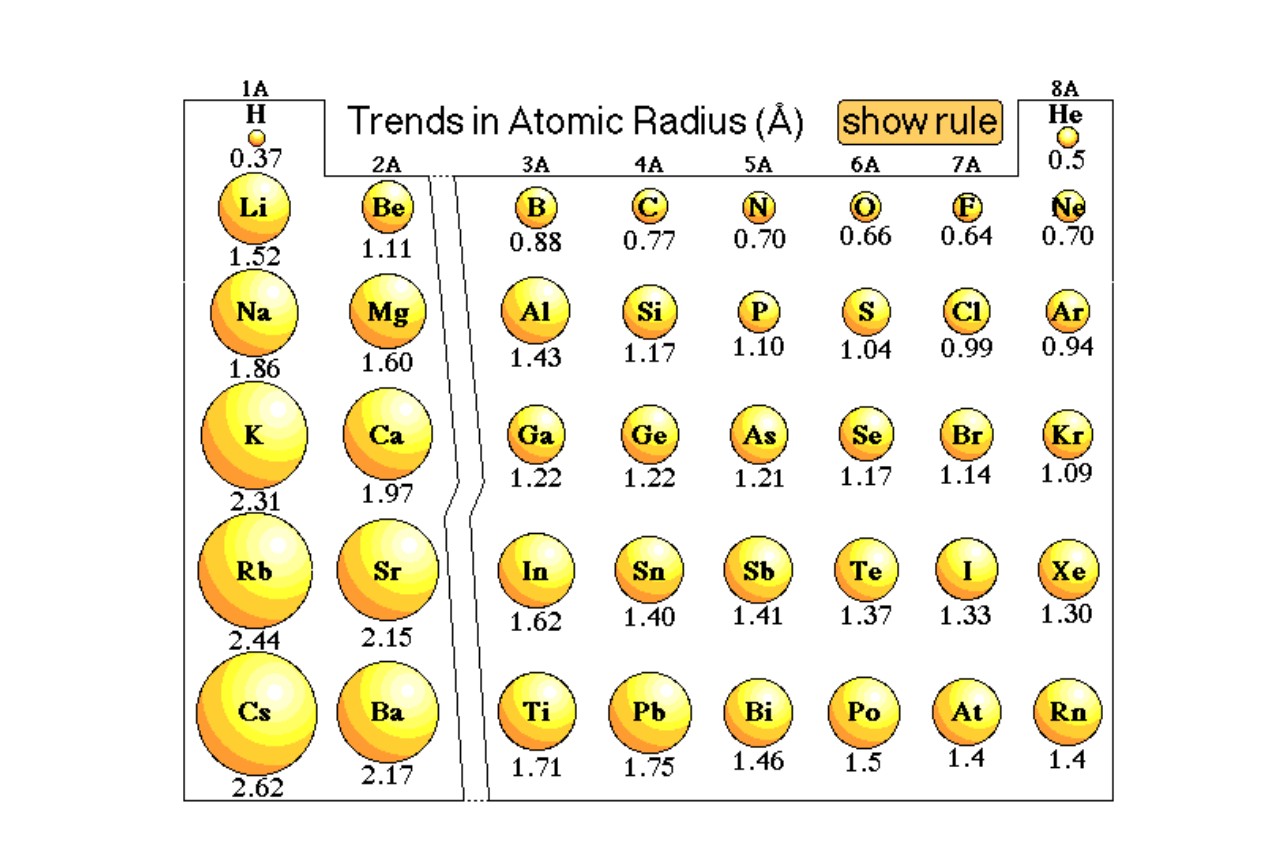
The effective atomic radius increases as you move down a group in the periodic table.
Within a group in the periodic table, the effective atomic radius tends to increase as you move from top to bottom. This is because each successive energy level is further from the nucleus, leading to an increase in size.
Effective atomic radius decreases as you move across a period in the periodic table.
As you move from left to right across a period in the periodic table, the effective atomic radius generally decreases. This is due to an increase in the positive charge of the nucleus, which attracts the electrons closer to the nucleus and reduces the overall size of the atom.
Effective atomic radius can also be influenced by the presence of neighboring atoms.
In a crystal lattice or a bonded structure, the effective atomic radius can be affected by the presence of neighboring atoms. The repulsion or attraction between atoms can cause the effective atomic radius to change.
The concept of effective atomic radius is widely used in predicting and explaining chemical reactions.
Chemists use the effective atomic radius to understand how atoms interact and bond with each other. It helps in predicting reactivity, solubility, and other chemical properties of elements and compounds.
Effective atomic radius is also important in determining the physical properties of materials.
The size of atoms affects the density, melting point, and conductivity of materials. By considering the effective atomic radius, scientists can gain insights into the physical properties of substances.
The atomic radius can be determined experimentally using X-ray crystallography.
X-ray crystallography is a powerful technique used to determine the arrangement of atoms within a crystal. By analyzing the diffraction patterns of X-rays, scientists can obtain information about the atomic radius of individual atoms.
The Pauling scale is often used to compare the effective atomic radii of different elements.
Linus Pauling, the renowned chemist, developed a scale to compare the effective atomic radii of elements. This scale allows scientists to make comparisons and identify trends in atomic size across the periodic table.
Effective atomic radius is influenced by the presence of lone pairs of electrons.
Lone pairs of electrons can occupy more space and cause the effective atomic radius to increase. This effect is particularly noticeable in elements that have a high electron density, such as nitrogen and oxygen.
The effective atomic radius can be estimated using various theoretical models.
Chemists have developed multiple theoretical models, such as the VSEPR theory and the molecular orbital theory, to estimate the effective atomic radius of atoms and ions. These models provide valuable insights into the size and shape of molecules.
In Conclusion
The effective atomic radius is a fundamental concept in chemistry that helps us understand the size and behavior of atoms. It plays a crucial role in predicting chemical reactions, determining the physical properties of materials, and explaining the periodic trends observed in the periodic table. By studying the effective atomic radius, scientists can unravel the mysteries of the microscopic world and make advancements in various fields, from materials science to pharmaceutical research.
Conclusion
Understanding the concept of effective atomic radius is crucial in the study of chemistry. It is not only a fundamental property of atoms but also plays a significant role in determining the chemical and physical behavior of elements. In this article, we have explored 12 intriguing facts about effective atomic radius, shedding light on its importance and application in various fields.
From the relationship between atomic size and electronegativity to the factors influencing effective atomic radius, we have delved into the intricacies of this concept. We have also learned about its connection with the periodic trend and its role in explaining chemical bonding and reactivity.
Furthermore, we have examined how effective atomic radius affects the properties of elements, such as ionization energy, metallic character, and atomic packing. By understanding these facts, scientists and researchers can make informed decisions in the design of new materials and the development of innovative technologies.
In conclusion, effective atomic radius is a fascinating concept that illuminates the intricate nature of atoms and their interactions. It serves as a valuable tool in predicting and understanding the behavior of elements, paving the way for advancements in the field of chemistry.
FAQs
1. What is effective atomic radius?
Effective atomic radius refers to the size of an atom, taking into account the influence of surrounding atoms and electron density distribution.
2. How is effective atomic radius different from atomic radius?
Atomic radius only considers the distance between the nucleus and the outermost electron, whereas effective atomic radius accounts for the size of the atom in a molecule or crystal lattice.
3. What factors affect the effective atomic radius?
The effective atomic radius is influenced by factors like electron-electron repulsion, nuclear charge, and electron shielding.
4. How does effective atomic radius affect chemical bonding?
The effective atomic radius influences the strength and nature of chemical bonds. Smaller atomic radius results in stronger bonds, while larger atomic radius leads to weaker bonds.
5. Is effective atomic radius the same for all elements?
No, the effective atomic radius varies from element to element due to variations in electron configuration and other atomic properties.
6. Can effective atomic radius be measured directly?
Effective atomic radius cannot be measured directly but is determined using theoretical calculations and experimental data.
7. How does effective atomic radius affect chemical reactivity?
Effective atomic radius influences how easily an atom can gain, lose, or share electrons, impacting its reactivity with other elements.
8. Can effective atomic radius change in different chemical environments?
Yes, the effective atomic radius can change in different chemical environments based on the surrounding atoms and the type of bonding.
9. Does effective atomic radius follow a periodic trend?
Yes, effective atomic radius generally increases from right to left and from top to bottom on the periodic table.
10. How is effective atomic radius measured in crystal structures?
In crystal structures, effective atomic radius can be measured by analyzing the distances between neighboring atoms in the lattice.
Exploring the intriguing world of effective atomic radius is just the beginning of your journey into the fascinating realm of chemistry. Unravel the mysteries of atomic structure, where protons, neutrons, and electrons dance in perfect harmony. Discover how periodic trends shape the behavior of elements across the table, from the smallest to the largest. Finally, prepare to be amazed by the power of electronegativity, the force that governs chemical bonding and reactivity. Embark on this thrilling adventure and expand your knowledge of the building blocks of our universe.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


