VSEPR (Valence Shell Electron Pair Repulsion) theory is a fundamental concept in chemistry that helps us understand the shapes of molecules and predict their molecular geometries. It provides a simple yet powerful framework for determining the spatial arrangement of atoms and electron pairs in a molecule by considering the repulsion between electron pairs. VSEPR theory not only allows chemists to visualize the three-dimensional shape of molecules but also plays a crucial role in explaining various chemical properties and reactivities.
In this article, we will explore 19 fascinating facts about VSEPR theory that will deepen your understanding of this essential concept. From the origins of the theory to its applications in predicting molecular geometries, we will uncover intriguing insights that highlight the significance and versatility of VSEPR theory in the field of chemistry. So, let’s dive right in and discover the amazing world of VSEPR theory!
Key Takeaways:
- VSEPR theory helps chemists predict the shapes of molecules by considering how electron pairs repel each other. This knowledge is crucial for understanding how different substances behave and interact with each other.
- VSEPR theory is like a superpower for chemists, allowing them to see into the invisible world of molecules and understand their shapes and properties. It’s a fundamental concept that shapes the way we study and create new substances.
VSEPR theory is based on the concept of electron repulsion.
According to VSEPR theory, electron pairs in the valence shell of an atom repel each other and try to maximize the distance between them.
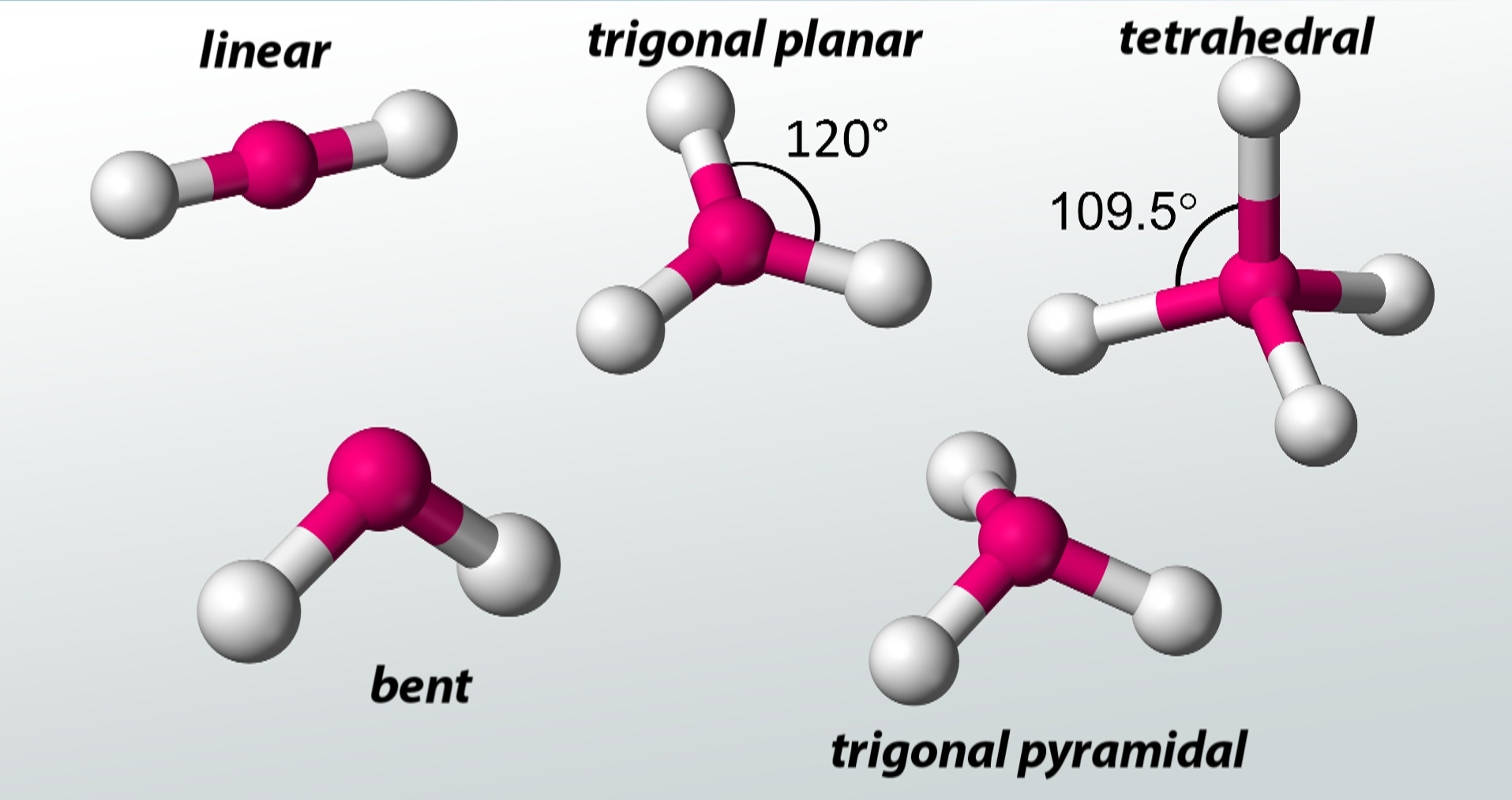
The central atom and surrounding atoms are considered in VSEPR theory.
In VSEPR theory, the shape of a molecule is determined by the arrangement of atoms and lone pairs around the central atom.
VSEPR theory can predict the shape of both simple and complex molecules.
Whether it’s a simple diatomic molecule or a complex organic compound, VSEPR theory can accurately predict their shapes.
VSEPR theory is based on the concept of electron pairs and the repulsion between them.
The theory assumes that electron pairs, whether they are bonding pairs or lone pairs, repel each other and distribute themselves as far apart as possible.
VSEPR theory is widely used in the field of organic chemistry.
Organic chemists rely on VSEPR theory to understand the three-dimensional structures of organic molecules, which is crucial for determining their reactivity and properties.
The basic premise of VSEPR theory is supported by experimental data.
Various experimental techniques, such as X-ray crystallography and spectroscopy, have confirmed the validity of VSEPR theory by directly observing and measuring molecular shapes.
The VSEPR model distinguishes between bonded and non-bonded electron pairs.
The theory considers the repulsion between bonding pairs and between lone pairs, resulting in different molecular geometries.
VSEPR theory explains the concept of bond angles.
By considering the repulsion between electron pairs, VSEPR theory provides a rationale for the observed bond angles in molecules.
VSEPR theory can predict the geometry of polyatomic ions.
Not only can VSEPR theory predict the molecular shape of neutral molecules, but it is also applicable to polyatomic ions, which play a crucial role in many chemical reactions.
VSEPR theory accounts for the effects of lone pairs on molecular shape.
Lone pairs of electrons affect the overall shape of a molecule and can lead to distortions in the bond angles away from the idealized values.
The repulsion between lone pairs of electrons is stronger than the repulsion between bonding pairs.
This stronger repulsion leads to specific geometries where lone pairs have more space around them, resulting in distortions in the molecular shape.
VSEPR theory is a useful tool in predicting the polarity of molecules.
The arrangement of atoms and lone pairs in a molecule, as predicted by VSEPR theory, provides insights into the overall polarity of the molecule.
VSEPR theory is not limited to covalent compounds.
While initially developed for understanding covalent compounds, VSEPR theory can also be applied to the shapes of ionic compounds and coordination complexes.
VSEPR theory is a fundamental concept in understanding molecular bonding and structure.
By providing insights into the three-dimensional arrangement of atoms, VSEPR theory forms the foundation for understanding the stability and reactivity of molecules.
VSEPR theory has practical applications in various scientific fields.
From drug development to materials science, the knowledge of molecular shape and structure obtained from VSEPR theory is essential for designing new compounds with specific properties.
VSEPR theory can be extended to complex molecules with multiple central atoms.
By considering each central atom separately and analyzing the arrangement of atoms and lone pairs around them, VSEPR theory can predict the overall shape of complex molecules.
VSEPR theory is not without limitations.
While VSEPR theory provides valuable insights into molecular shapes, it does not account for all aspects of molecular behavior, such as the effects of orbital hybridization and molecular vibrations.
VSEPR theory continues to be a topic of research and refinement.
Scientists are continuously expanding and refining the principles of VSEPR theory to accurately predict the structures and shapes of increasingly complex molecules.
VSEPR theory is an essential tool in the education of chemistry students.
The understanding of VSEPR theory is a fundamental aspect of chemistry education, as it forms the basis for further exploration into molecular structure and bonding.
In conclusion, VSEPR theory is a powerful concept in chemistry that allows us to predict the three-dimensional shape of molecules. Its wide application in various scientific fields highlights its importance in understanding molecular structure and reactivity. By considering the repulsion between electron pairs, VSEPR theory provides valuable insights into the geometries of molecules, enabling scientists to design new compounds and study their properties. With ongoing research and refinement, VSEPR theory continues to revolutionize our understanding of molecular shapes and advance the field of chemistry as a whole.
Conclusion
VSEPR theory, short for Valence Shell Electron Pair Repulsion theory, is a fundamental concept in chemistry that helps us understand the shape and structure of molecules. Developed by Ronald Gillespie and Ronald Nyholm in the 1950s, this theory has revolutionized the way we visualize and predict the geometry of molecules.
Through the application of VSEPR theory, scientists can determine the arrangement of electron pairs around the central atom in a molecule, which directly influences its spatial structure. This theory is based on the principle that electron pairs in the valence shell of an atom repel each other, resulting in specific molecular shapes.
By understanding VSEPR theory, chemists can not only explain why certain molecules have particular shapes but also predict the reactivity, polarity, and other physical properties of compounds. This knowledge is crucial in various fields, including pharmaceuticals, materials science, and environmental chemistry.
In conclusion, VSEPR theory is an invaluable tool that allows scientists to comprehend the three-dimensional nature of molecules and provides insights into their properties and behavior.
FAQs
Q: What does VSEPR theory stand for?
A: VSEPR stands for Valence Shell Electron Pair Repulsion theory.
Q: Who developed the VSEPR theory?
A: The VSEPR theory was developed by Ronald Gillespie and Ronald Nyholm in the 1950s.
Q: Why is VSEPR theory important?
A: VSEPR theory is important because it helps us understand the three-dimensional structure and shape of molecules, which in turn affects their chemical properties and behavior.
Q: How does VSEPR theory predict molecular geometry?
A: VSEPR theory predicts molecular geometry by considering the repulsion between electron pairs in the valence shell of an atom. The shapes of molecules are determined by minimizing this electron pair repulsion.
Q: What is the significance of VSEPR theory in chemistry?
A: VSEPR theory allows chemists to predict and explain the shapes of molecules, which is crucial for understanding their reactivity, polarity, and other physical properties. It is widely used in various branches of chemistry.
Intrigued by the captivating world of molecular geometry? Continue exploring the fascinating principles behind chemical bonding and structure. Delve deeper into the intricacies of Valence Shell Electron Pair Repulsion (VSEPR) theory, uncovering extraordinary facts that shed light on its significance. From predicting molecular shapes to understanding polarity, VSEPR theory holds the key to unlocking the secrets of chemical compounds. Embark on a journey through the realm of chemistry, where VSEPR theory serves as a guiding light, illuminating the path to scientific discovery and innovation.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
