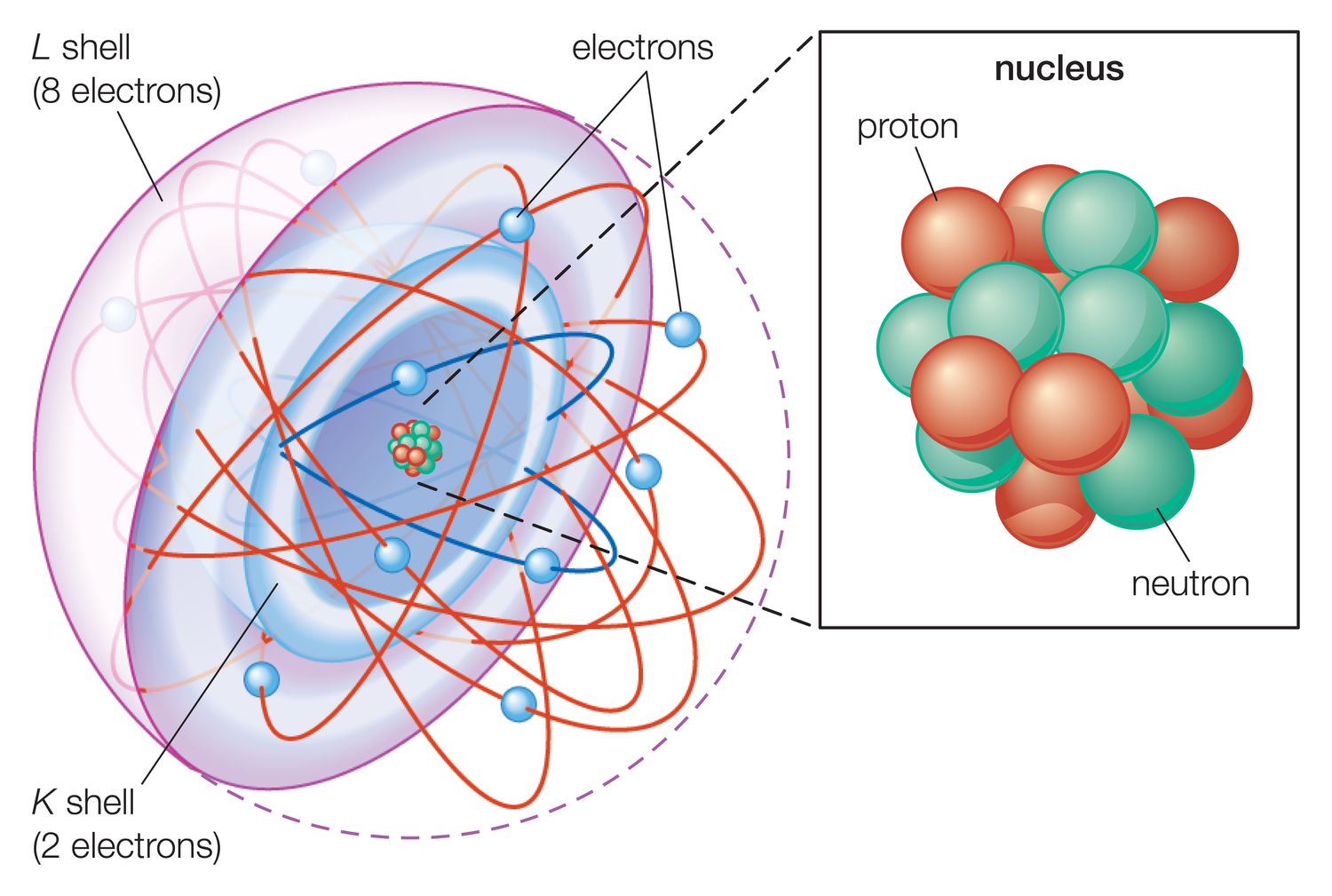
When it comes to understanding the arrangement of electrons in an atom, the Aufbau principle is a fundamental concept in chemistry. This principle, also known as the building-up principle, provides valuable insights into the organization of electrons in atomic orbitals. By following this principle, scientists can determine the electron configurations of various elements and predict their chemical behavior.
The Aufbau principle will be explored in this article, where we will delve into 9 intriguing facts that shed light on this important principle. From its historical origins to its applications in understanding atomic structure, the Aufbau principle has revolutionized our understanding of the behavior of electrons in atoms. So let’s dive in and uncover the fascinating world of the Aufbau principle!
Key Takeaways:
- The Aufbau Principle explains how electrons fill up an atom’s orbitals, influencing its chemical reactivity and properties. It’s like following a specific order when arranging your toys in a toy box!
- Understanding the Aufbau Principle helps scientists predict how electrons are arranged in elements and how they behave in the periodic table. It’s like solving a puzzle to uncover the secrets of the elements!
The Aufbau Principle Explains Electron Distribution
The Aufbau Principle states that electrons fill the lowest energy orbital available before moving to higher-energy orbitals. This sequential filling of electron orbitals is essential in determining the electron configuration of an atom and influences its chemical reactivity.
Electrons Follow the Building-Up Order
According to the Aufbau Principle, electrons occupy the atomic orbitals in a specific order. This order is commonly referred to as the “building-up” order, where orbitals with lower principal quantum numbers (n) are filled first, followed by higher principal quantum numbers.
Aufbau Principle Determines Periodic Table Structure
The Aufbau Principle plays a fundamental role in organizing the elements within the periodic table. By following the principle, the periodic table is structured in a way that elements within the same group or period have similar electron configurations and exhibit comparable chemical properties.
Exceptions to the Aufbau Principle Exist
While the Aufbau Principle provides a useful framework, certain exceptions exist due to electron-electron repulsion and energy considerations. For example, elements such as chromium and copper deviate from the expected electron configuration due to the stability gained from half-filled or fully filled d orbitals.
Quantum Mechanics Explains the Aufbau Principle
The Aufbau Principle is rooted in the principles of quantum mechanics, specifically the Pauli Exclusion Principle and Hund’s Rule. These principles describe the behavior and organization of electrons within an atom, leading to the derivation of the Aufbau Principle.
Electron Configuration Determines Element Properties
The electron configuration resulting from the Aufbau Principle greatly influences the chemical and physical properties of elements. The arrangement of electrons in different energy levels and orbitals determines the element’s reactivity, stability, and bonding behavior.
Aufbau Principle Applies to Multielectron Atoms
The Aufbau Principle is not limited to hydrogen and helium, as it also applies to multielectron atoms. For atoms with multiple electrons, the principle guides the filling of both the inner and outer electron shells according to the building-up order.
Spin and Orbital Quantum Numbers Determine Aufbau Order
The Aufbau order is determined by the spin and orbital quantum numbers of electrons. Electrons with different spin orientations and orbital shapes occupy different energy levels, leading to the sequential filling pattern dictated by the Aufbau Principle.
Aufbau Principle Enables Prediction of Electron Configurations
By applying the Aufbau Principle, scientists can predict the electron configurations of different elements and determine their placement within the periodic table. This understanding allows for the identification of trends and patterns in chemical behavior down the periodic table.
In conclusion, the Aufbau Principle is a fundamental concept in chemistry that governs the order of electron filling in atoms. By understanding this principle, scientists can decipher the electron configurations of elements and unravel their chemical behavior. The 9 intriguing facts about the Aufbau Principle highlight its significance in understanding the structure and properties of matter.
Conclusion
In conclusion, the Aufbau Principle is a fundamental concept in Chemistry that helps us understand the arrangement of electrons in an atom. It provides a systematic approach to filling the electron orbitals based on their energy levels. By following the Aufbau Principle, scientists can predict the electronic configuration of different elements and explain various chemical properties.
The nine intriguing facts about the Aufbau Principle that we have explored shed light on its significance and interesting aspects. From the concept of electron filling order to exceptions and special cases, understanding these facts deepens our knowledge of atomic structure and chemical behavior.
By recognizing the patterns and exceptions encoded in the Aufbau Principle, scientists can unlock new insights into the behavior of elements and compounds. As we continue to explore the fascinating world of Chemistry, the Aufbau Principle remains a cornerstone of our understanding of the atomic world.
FAQs
1. What is the Aufbau Principle?
The Aufbau Principle is a fundamental principle in Chemistry that explains the arrangement of electrons in an atom. It states that electrons fill the lowest energy levels available before moving to higher energy levels.
2. How does the Aufbau Principle determine electron configurations?
The Aufbau Principle determines electron configurations by following a specific order of filling electron orbitals based on their increasing energy levels. It starts with the lowest energy level and fills up the subsequent levels until all electrons are assigned to their respective orbitals.
3. Are there any exceptions to the Aufbau Principle?
Yes, there are some exceptions to the Aufbau Principle. For example, elements like chromium and copper deviate from the expected electron configuration due to stability considerations. They have a half-filled or completely filled d subshell, which is more stable than the expected configuration.
4. How does the Aufbau Principle contribute to understanding chemical properties?
By determining the electron configuration, the Aufbau Principle helps us understand the distribution of electrons in an atom, which in turn influences the chemical properties of elements. It provides insights into factors like ionization energy, electron affinity, and reactivity.
5. Can the Aufbau Principle predict the behavior of all elements?
Although the Aufbau Principle is a useful tool for predicting the behavior of most elements, there are exceptions and complexities that require further exploration. Scientists continue to study and refine our understanding of electron configuration and its impact on chemical behavior.
Aufbau Principle provides a fascinating foundation for understanding atomic structure, but there's so much more to explore! Dive into the world of electron configuration notation and uncover surprising facts that will deepen your knowledge. Ready to have your mind blown? Prepare to be amazed by quantum mechanics and its unbelievable implications. And if you thought you knew everything about atomic structure, think again – enigmatic facts await your discovery. Keep learning and let your curiosity guide you through the captivating realm of chemistry!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
