The Pauli Exclusion Principle is one of the fundamental concepts in quantum mechanics that has revolutionized our understanding of the behavior of particles. Named after the brilliant physicist Wolfgang Pauli, this principle states that no two identical fermions can occupy the same quantum state simultaneously. In simpler terms, it means that particles such as electrons in an atom must have unique sets of quantum numbers, leading to the observed stability of matter. This principle plays a crucial role in determining the electronic structure of atoms, the behavior of electrons in solids, and even the formation of white dwarfs and neutron stars. In this article, we delve into 16 captivating facts about the Pauli Exclusion Principle that highlight its significance and shed light on the intriguing world of quantum mechanics.
Key Takeaways:
- The Pauli Exclusion Principle, formulated by Wolfgang Pauli, explains why electrons in atoms behave the way they do, leading to the stability of matter and the structure of the periodic table.
- This principle is super important in quantum mechanics and affects everything from the behavior of stars to the properties of superconducting materials. It’s like a rule that electrons follow to keep everything in the universe in order!
The Pauli Exclusion Principle was formulated by Wolfgang Pauli.
Named after its creator, Wolfgang Pauli, this fundamental principle in quantum mechanics states that no two identical fermions can occupy the same quantum state within a quantum system concurrently.
It is a cornerstone of quantum mechanics.
The Pauli Exclusion Principle is one of the key principles that govern quantum mechanics, playing a crucial role in understanding the behavior and properties of electrons.
It applies to all fermions.
Fermions, a class of particles that includes electrons, protons, and neutrons, must adhere to the Pauli Exclusion Principle.
It contributes to the stability of matter.
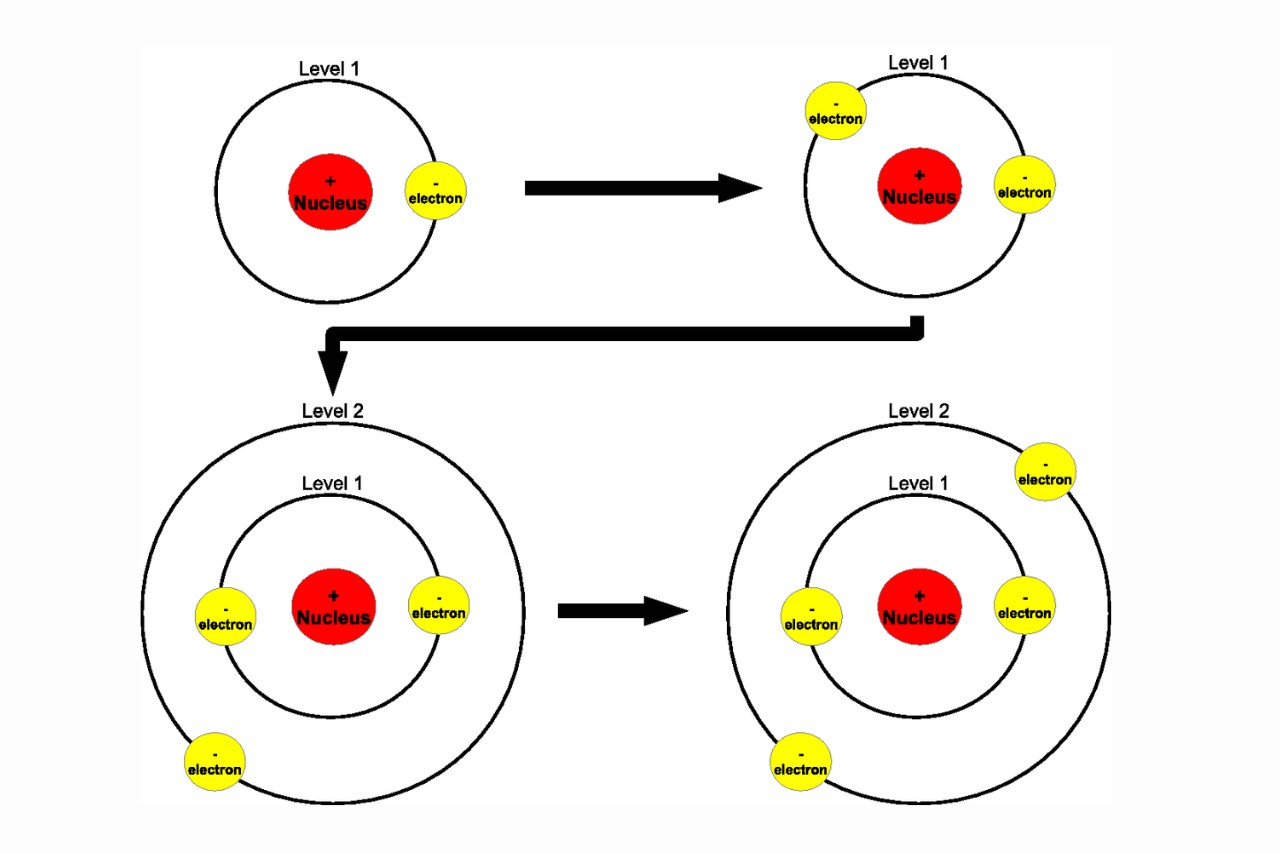
The Pauli Exclusion Principle prevents electrons from occupying the same energy level and spatial location within an atom, leading to distinct energy levels and the stability of matter.
It explains the periodic table.
The Pauli Exclusion Principle dictates that no more than two electrons can occupy the same orbital, providing a basis for the structure and organization of the elements in the periodic table.
It has wide-ranging applications.
The Pauli Exclusion Principle is applied in various fields, including condensed matter physics, astrophysics, and even nuclear physics.
It is related to electron spin.
The Pauli Exclusion Principle is intimately linked to the concept of electron spin, which can have two possible states: spin-up and spin-down.
It prohibits electron degeneracy pressure in white dwarf stars.
In massive white dwarf stars, the Pauli Exclusion Principle prevents electrons from occupying the same quantum state, counteracting the force of gravity and preventing further collapse.
It played a key role in the development of quantum field theory.
The Pauli Exclusion Principle heavily influenced the formulation of quantum field theory, a theoretical framework that combines quantum mechanics and special relativity.
It allows for the existence of neutron stars.
The Pauli Exclusion Principle helps support the tremendous gravitational pressure in neutron stars by preventing neutrons from occupying the same quantum state.
It was first proposed in 1925.
Wolfgang Pauli formulated the exclusion principle in 1925 to explain the electronic structure of atoms and the behavior of electrons.
It accounts for the chemical properties of elements.
The Pauli Exclusion Principle is responsible for the distinct chemical behavior and properties of different elements due to the arrangement and filling of electron orbitals.
Violation of the Pauli Exclusion Principle could lead to strange phenomena.
If the Pauli Exclusion Principle were to be violated, it could result in bizarre scenarios like the collapse of matter or the breakdown of atomic structure.
It was initially met with skepticism.
When Wolfgang Pauli proposed his exclusion principle, it faced initial resistance and skepticism. However, subsequent experimental evidence supported its validity.
It has implications for superconductivity.
The Pauli Exclusion Principle places restrictions on how electrons can pair up in superconducting materials, influencing their behavior and properties.
It is a consequence of quantum indistinguishability.
The Pauli Exclusion Principle arises from the quantum mechanical concept of particle indistinguishability, which states that identical particles cannot be distinguished from each other.
Conclusion
The Pauli Exclusion Principle is a fundamental concept in quantum mechanics that has revolutionized our understanding of the behavior of particles. It states that no two identical fermions can occupy the same quantum state simultaneously. This principle, developed by Wolfgang Pauli in 1925, has had significant implications in various fields of science, from chemistry to astrophysics.The Pauli Exclusion Principle helps explain the stability of matter and the formation of elements. Without it, electrons would all occupy the lowest energy levels, resulting in a collapse of atomic structure. This principle also leads to the concept of electron shells and configurations, which determine the chemical properties of elements and the formation of chemical bonds.Furthermore, the Pauli Exclusion Principle has been crucial in understanding exotic states of matter, such as white dwarfs, neutron stars, and even the behavior of quarks in subatomic particles. Its wide-ranging impact on our understanding of the universe makes it a captivating topic for further exploration and study.
FAQs
1. Why is the Pauli Exclusion Principle important in chemistry?
The Pauli Exclusion Principle is vital in understanding the electronic structure of atoms and the formation of chemical bonds. It helps explain the stability of matter and why certain elements exhibit specific chemical properties.
2. How does the Pauli Exclusion Principle affect electron configurations?
The Pauli Exclusion Principle dictates that no two electrons in an atom can have the same set of quantum numbers. This results in the organization of electrons into distinct energy levels, known as electron shells, and determines the arrangement of electrons in orbitals within those shells.
3. Can you give an example of the Pauli Exclusion Principle in action?
One example is the filling of electron orbitals in atoms. Each orbital can hold a maximum of two electrons, with opposite spins. The Pauli Exclusion Principle ensures that electrons occupy different orbitals before doubling up in the same orbital.
4. Does the Pauli Exclusion Principle apply to all particles?
No, the Pauli Exclusion Principle specifically applies to fermions, which are particles that obey Fermi-Dirac statistics. Fermions include electrons, protons, and neutrons. Bosons, on the other hand, do not follow this principle and can occupy the same quantum state simultaneously.
5. How does the Pauli Exclusion Principle impact the behavior of matter under extreme conditions?
Under extreme conditions, such as high pressures or temperatures, matter can undergo phase transitions and exhibit unique properties. The Pauli Exclusion Principle plays a crucial role in these phenomena by governing the behavior of particles and determining the states of matter that can exist.
Pauli's exclusion principle continues captivating physicists, chemists, astronomers alike. This fundamental concept quantum mechanics explains diverse phenomena, from electron behavior atoms to neutron stars' existence. While we've explored 16 facts about Pauli exclusion principle, there's still more to learn. Why not satisfy your curiosity by reading 15 unbelievable facts about Pauli exclusion principle? You'll be amazed at how this principle shapes our understanding of the universe.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


