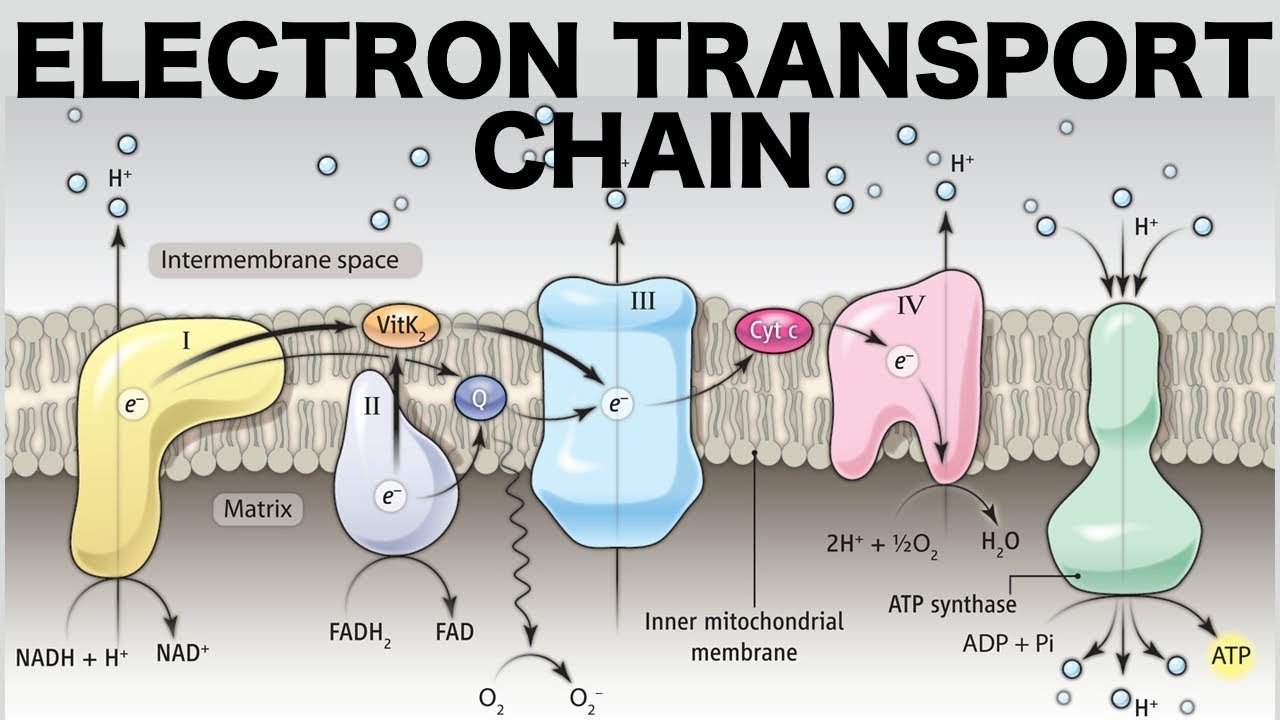
The electron transport chain (ETC) complexes are an essential component of cellular respiration, playing a crucial role in producing ATP, the energy currency of cells. These complexes are found in the inner mitochondrial membrane and are responsible for the transfer of electrons from electron donors to electron acceptors, resulting in the generation of a proton gradient that drives ATP synthesis.
While the ETC complexes may sound complex, they also hold a fascinating world of biology within them. From their intricate structure to their vital role in aerobic respiration, there are plenty of intriguing facts to discover. In this article, we’ll delve into 15 captivating facts about these electron transport chain complexes, shedding light on their significance and unveiling some little-known aspects of their functioning. So, let’s embark on this journey to explore the inner workings of a remarkable cellular process!
Key Takeaways:
- The electron transport chain is like a power plant inside our cells, generating energy (ATP) for all our activities. It’s a crucial process for keeping us alive and kicking!
- If the electron transport chain gets disrupted, it can lead to diseases and affect how we age. Scientists study it to understand how our bodies work and why we get sick.
Electron Transport Chain Complexes play a vital role in cellular respiration.
The electron transport chain is a series of protein complexes located in the inner membrane of mitochondria that generate ATP, the energy currency of cells. This process is crucial for the production of energy that fuels various cellular activities.
There are five major protein complexes involved in the electron transport chain.
The complexes are labeled complex I to complex V. Each complex has a specific function in the sequential transfer of electrons and protons, contributing to the generation of ATP.
Complex IV, also known as cytochrome c oxidase, is responsible for the final step of electron transfer.
Complex IV transfers electrons from cytochrome c to molecular oxygen, resulting in the formation of water. This step is essential for the completion of the electron transport chain.
The electron transport chain occurs in the inner membrane of mitochondria.
The inner membrane of mitochondria provides a highly specialized environment for the electron transport chain complexes to function efficiently. This membrane is impermeable to ions, allowing the establishment of an electrochemical gradient that drives ATP synthesis.
Electron transport chain complexes are composed of both protein and non-protein components.
Protein subunits make up the majority of the complexes, while non-protein components such as coenzymes and prosthetic groups are essential for electron transfer and enzymatic activity.
NADH and FADH2 are key electron carriers in the electron transport chain.
Electrons from NADH and FADH2 are transferred to the first complex of the electron transport chain, initiating the flow of electrons through the protein complexes.
The transfer of electrons in the electron transport chain is coupled with the pumping of protons.
As electrons flow through the protein complexes, protons are pumped from the mitochondrial matrix to the intermembrane space, creating an electrochemical gradient.
The electron transport chain is regulated by ATP production and the energy needs of the cell.
When ATP levels are high, the electron transport chain slows down, reducing the proton gradient. Conversely, when ATP levels are low, the chain speeds up to generate more ATP.
Reactive oxygen species (ROS) can be generated during electron transport.
The transfer of electrons within the chain can result in the formation of ROS, which can cause oxidative damage to cells. Antioxidant defense systems help mitigate this damage.
Inhibitors of the electron transport chain can disrupt ATP production.
Various substances, such as certain drugs and toxins, can interfere with the function of electron transport chain complexes, leading to impaired ATP synthesis and cellular dysfunction.
The electron transport chain is an aerobic process.
Oxygen is the final electron acceptor in the chain, without which the entire process would be stalled, halting ATP production.
The electron transport chain is highly efficient at generating ATP.
Compared to other metabolic pathways, the electron transport chain has a high energy conversion efficiency, making it a crucial player in cellular energy production.
The electron transport chain is susceptible to damage from oxidative stress.
Oxidative stress, caused by an imbalance between the production of ROS and the antioxidant defense system, can impair the function of electron transport chain complexes and disrupt ATP synthesis.
Disorders of the electron transport chain can lead to mitochondrial diseases.
Genetic mutations or defects in the electron transport chain complexes can result in mitochondrial diseases, which can affect various organs and tissues due to the vital role of mitochondria in energy production.
Research on the electron transport chain contributes to the understanding of aging and disease.
Understanding the intricacies of the electron transport chain and its role in cellular function provides insights into the processes of aging and the development of diseases associated with mitochondrial dysfunction.
Conclusion
Electron transport chain complexes play a crucial role in the process of cellular respiration, allowing cells to generate energy in the form of ATP. These complex structures are fascinating in their intricate mechanisms and vital for the functioning of all living organisms.Through a series of redox reactions, electron transport chain complexes transfer electrons from electron donors to electron acceptors, creating a proton gradient across the inner mitochondrial membrane. This gradient drives ATP synthesis through ATP synthase, resulting in the production of energy that powers various cellular activities.Understanding the intricacies of electron transport chain complexes not only provides insights into fundamental biological processes but also holds significant implications for fields like medicine and biochemistry. Further research into these complexes may uncover new ways to combat diseases or develop more efficient strategies for energy production.In conclusion, the electron transport chain complexes offer a captivating glimpse into the underlying mechanisms that fuel life. Exploring their complexities sheds light on the marvels of cellular respiration and broadens our knowledge of the intricate workings of living organisms.
FAQs
1. What are electron transport chain complexes?
Electron transport chain complexes are protein structures located in the inner mitochondrial membrane that facilitate the transfer of electrons during cellular respiration. They are essential for the production of ATP, the energy currency in cells.
2. How many electron transport chain complexes are there?
There are four main complexes in the electron transport chain: complex I (NADH dehydrogenase), complex II (succinate dehydrogenase), complex III (cytochrome bc1 complex), and complex IV (cytochrome c oxidase).
3. What is the role of electron transport chain complexes in ATP synthesis?
Electron transport chain complexes help create a proton gradient across the inner mitochondrial membrane. This gradient drives ATP synthesis through the action of ATP synthase, leading to the production of ATP molecules.
4. How do electron transport chain complexes function?
Electron transport chain complexes transfer electrons from electron donors, such as NADH and FADH2, to electron acceptors, such as oxygen. The electrons pass through the complexes in a series of redox reactions, releasing energy that is used to pump protons across the membrane.
5. Why are electron transport chain complexes important?
Electron transport chain complexes are vital for cellular respiration and energy production. They help convert the energy stored in food molecules into ATP, which is used by cells for various biological processes.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
