Electron affinity is a fundamental concept in chemistry that refers to the energy change that occurs when an atom or molecule gains an electron. It plays a crucial role in understanding various chemical reactions and the behavior of elements in the periodic table. While electron affinity may seem like a complex topic, it is also home to fascinating and extraordinary facts that shed light on the behavior of matter in the microscopic world. In this article, we will explore 20 extraordinary facts about electron affinity that will not only deepen your understanding of this concept but also uncover some mind-blowing insights into the world of chemistry. So, let’s dive in and discover the hidden wonders of electron affinity!
Key Takeaways:
- Electron affinity determines how likely an atom is to gain an electron and form stable compounds. It’s like a magnet that attracts electrons and influences chemical reactions.
- The periodic table helps us see patterns in electron affinity. Elements with higher electron affinity form stronger bonds, while noble gases are like “no thanks, I’m already stable!”
What is Electron Affinity?
Electron affinity is defined as the energy change when a neutral atom in the gaseous state acquires an electron to form a negatively charged ion.
Electron Affinity Trends
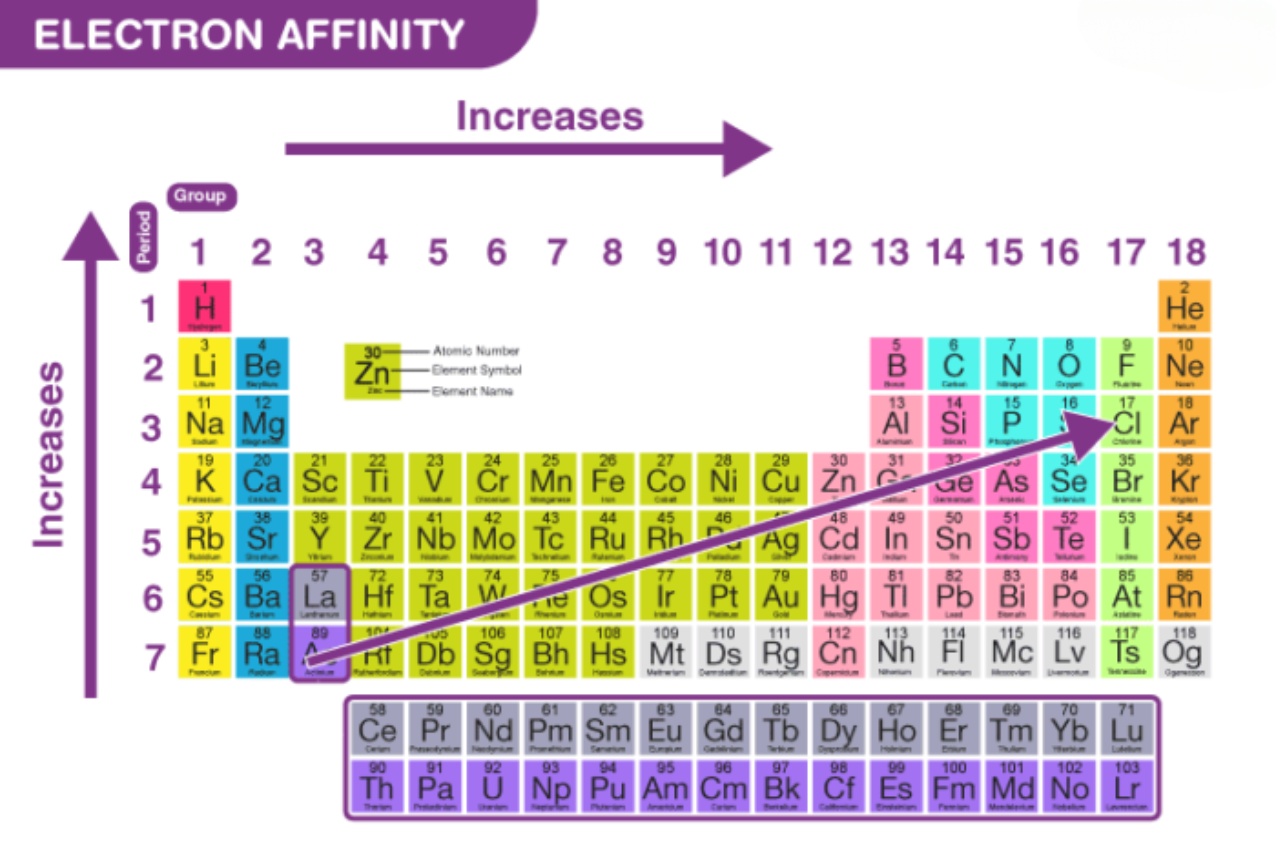
Electron affinity tends to increase from left to right across a period in the periodic table. However, there are exceptions to this trend due to factors such as atomic size and electron shielding.
Noble Gases and Electron Affinity
Noble gases have very low electron affinities since their electron shells are already complete, making them highly stable and unreactive.
Nonmetals vs. Metals
Nonmetals generally have higher electron affinities compared to metals. This is because nonmetals have a greater tendency to gain electrons and form negative ions.
Halogens and Electron Affinity
The halogens, such as fluorine and chlorine, have the highest electron affinities among all the elements. They readily accept an extra electron to achieve a stable configuration.
Alkali Metals and Electron Affinity
Alkali metals, such as lithium and sodium, have low electron affinities due to their large atomic size and low nuclear charge.
Electron Affinity and Ionic Bonding
Electron affinity plays a crucial role in the formation of ionic compounds. When atoms with different electron affinities come together, one atom may transfer an electron to the other to achieve a stable configuration.
Relationship with Ionization Energy
The ionization energy is the energy required to remove an electron from an atom, while electron affinity is the energy released when an atom gains an electron. These two properties are inversely related.
Factors Affecting Electron Affinity
Several factors influence the electron affinity of an atom, including its atomic size, nuclear charge, and electron configuration.
Electron Affinity and Chemical Reactivity
The electron affinity of an atom or ion influences its chemical reactivity. Elements with higher electron affinities are more likely to form stable compounds and engage in chemical reactions.
Measurement of Electron Affinity
Electron affinity is typically measured in electron volts (eV) or kilojoules per mole (kJ/mol). Experimental techniques such as electron capture spectroscopy are used to determine electron affinity values.
Negative and Positive Electron Affinities
Some elements exhibit negative electron affinities, indicating that more energy is required to add an electron to the system. On the other hand, positive electron affinities indicate the release of energy upon electron gain.
Electron Affinity and Periodic Trends
Electron affinity generally increases from left to right across a period in the periodic table, with the exception of noble gases and some transition metals.
Electron Affinity and Atomic Size
As the atomic size decreases, the electron affinity increases. This is due to the increased attraction between the positively charged nucleus and the incoming electron.
Electron Affinity and Group Trends
Within a group or family in the periodic table, electron affinity decreases as you move down the group. The increased atomic size and electron shielding result in a reduced ability to attract additional electrons.
Electron Affinity and Bond Strength
The electron affinity of an atom influences the strength of chemical bonds. Greater electron affinity leads to stronger ionic or covalent bonds.
Variation in Electron Affinity Values
Electron affinity values can vary depending on the experimental conditions, such as temperature, pressure, and the presence of other atoms or molecules.
Electron Affinity and Electron Configurations
The electron affinity of an atom is influenced by its electron configuration. Elements with partially filled or half-filled orbitals tend to have higher electron affinities.
Applications of Electron Affinity
Knowledge of electron affinity is crucial in various fields, including materials science, catalysis, and the study of chemical reactions.
Electron Affinity and Periodic Table
The periodic table provides a systematic way to analyze and compare the electron affinities of different elements. It helps in understanding the trends and patterns in electron affinity values.
These 20 extraordinary facts about electron affinity highlight its significance in understanding the behavior of elements and their chemical properties. Whether it’s the role of electron affinity in bond formation or the periodic trends observed, electron affinity continues to be a fascinating concept in the world of chemistry.
Conclusion
In conclusion, electron affinity is a fascinating concept in chemistry that plays a significant role in understanding the behavior of elements and their chemical reactions. Through exploring these 20 extraordinary facts about electron affinity, we have gained valuable insights into the intricacies of this fundamental concept.From its definition as the measure of an atom’s ability to attract and hold onto electrons, to its role in determining the stability and reactivity of elements, electron affinity provides a deeper understanding of the periodic table. We have learned about the trends in electron affinity across the periodic table, such as the general increase from left to right and the decrease down a group.Furthermore, we have explored the factors that influence electron affinity, such as atomic size, nuclear charge, and electron shielding. We have also discovered some exceptional cases where electron affinity deviates from the expected trends, highlighting the complexities of this phenomenon.Overall, electron affinity serves as a crucial tool in predicting and explaining chemical behavior. By delving into these extraordinary facts, we have unveiled some of the mysteries surrounding electron affinity, enhancing our understanding of the intricate world of chemistry.
FAQs
1. What is electron affinity?
Electron affinity is the measure of an atom’s ability to attract and hold onto electrons.
2. How is electron affinity measured?
Electron affinity is typically measured in electron volts (eV) or kilojoules per mole (kJ/mol).
3. What are the trends in electron affinity across the periodic table?
In general, electron affinity increases from left to right across a period and decreases down a group.
4. How does atomic size affect electron affinity?
Smaller atoms generally have higher electron affinities because their valence electrons are closer to the nucleus.
5. What factors influence electron affinity?
Factors such as atomic size, nuclear charge, and electron shielding can influence electron affinity.
6. Are there any exceptions to the trends in electron affinity?
Yes, there are some exceptional cases where electron affinity deviates from the expected trends, such as with elements like helium and noble gases.
7. How does electron affinity impact chemical reactions?
Electron affinity directly affects the stability and reactivity of elements and plays a crucial role in determining the likelihood and nature of chemical reactions.
8. Can electron affinity be negative?
Yes, electron affinity can be negative if the addition of an electron to an atom is energetically unfavorable.
9. How does electron affinity relate to ionization energy?
Ionization energy and electron affinity are closely related. Ionization energy is the energy required to remove an electron, while electron affinity is the energy released when an electron is added.
10. Are there any practical applications of electron affinity?
Understanding electron affinity is crucial in fields such as materials science, chemistry, and energy research, as it helps us design and manipulate substances for specific purposes.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
