Pi molecular orbitals are a fascinating concept in the realm of chemistry, and understanding their properties and characteristics can deepen our understanding of various chemical phenomena. These molecular orbitals play a crucial role in determining the stability, reactivity, and electronic properties of organic compounds and conjugated systems.
In this article, we will explore 12 surprising facts about pi molecular orbitals. From their formation and energy levels to their influence on aromaticity and delocalization, these facts shed light on the intricate world of molecular bonding and electron distribution. Whether you are a chemistry enthusiast or a student delving into the world of organic chemistry, these facts will surely captivate your interest and expand your knowledge of pi molecular orbitals.
Key Takeaways:
- Pi molecular orbitals are like special electron clouds that help make certain chemicals stable and useful in electronics. They follow rules and play a big role in many different areas of chemistry.
- Understanding pi molecular orbitals helps scientists make better drugs, electronic devices, and materials. They are like secret keys that unlock new possibilities in the world of chemistry.
The Concept of Pi Molecular Orbital
Pi molecular orbitals (? orbitals) are a type of molecular orbital that form from the overlapping of p orbitals in a molecule. They play a crucial role in organic chemistry, particularly in the bonding and stability of aromatic compounds.
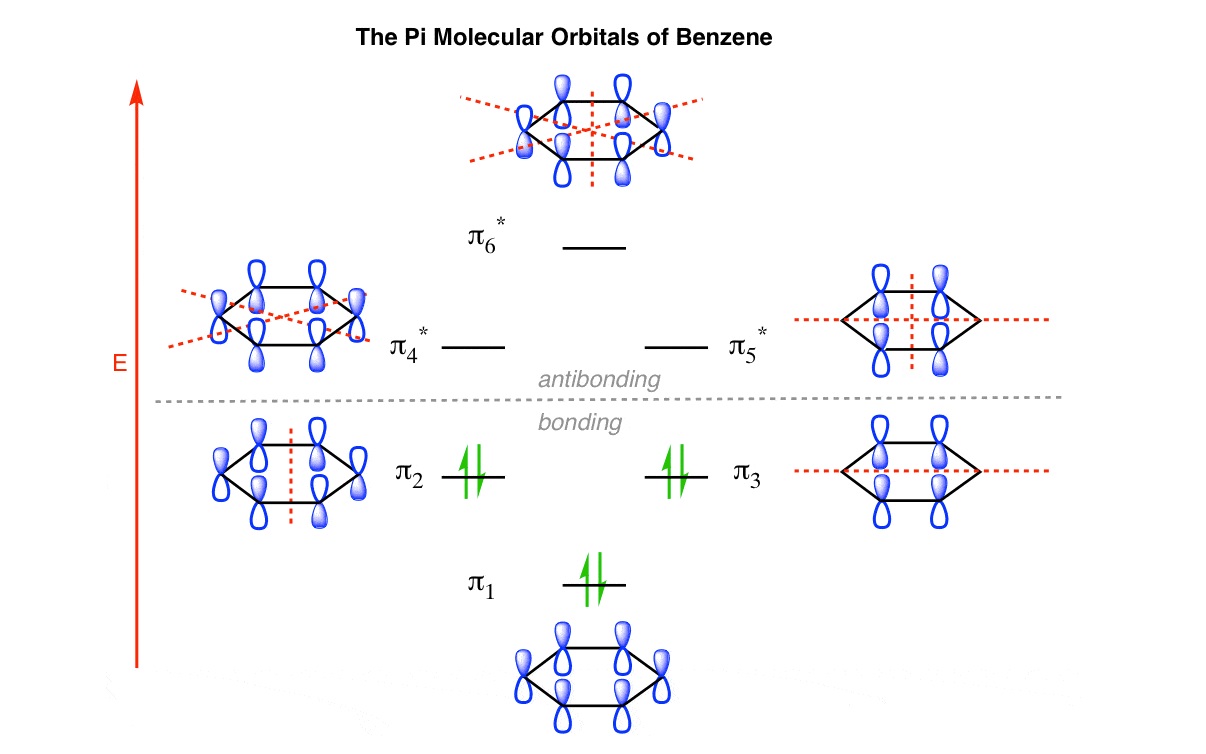
Pi Molecular Orbitals in Benzene
Benzene, a well-known aromatic compound, is characterized by the presence of six pi molecular orbitals. These orbitals form a ring-like structure above and below the benzene molecule, contributing to its unique stability.
Overlapping of P Orbitals
The formation of pi molecular orbitals occurs when the p orbitals of adjacent atoms align and overlap. This overlap allows for the delocalization of electrons, resulting in the formation of multiple bonding and antibonding orbitals.
Different Energy Levels of Pi Molecular Orbitals
Pi molecular orbitals can have different energy levels depending on their bonding or antibonding nature. The bonding pi orbitals, known as pi bonding orbitals, have lower energy levels, while the antibonding pi orbitals have higher energy levels.
Contribution to Aromaticity
The presence of pi molecular orbitals in aromatic compounds is one of the key factors contributing to their stability and aromaticity. The delocalization of electrons within these orbitals creates a “resonance” effect, which enhances the compound’s stability.
Extended Conjugation in Pi Systems
Pi molecular orbitals can extend beyond individual molecules and involve multiple conjugated systems. This extended conjugation imparts unique electronic and optical properties to the compounds, making them useful in various applications.
Role in Electron Delocalization
One of the significant roles of pi molecular orbitals is to facilitate the delocalization of electrons. This delocalization allows for better conductivity in certain materials and plays a crucial role in electron transfer reactions.
Pi Molecular Orbitals in Organic Electronics
The understanding and manipulation of pi molecular orbitals have paved the way for advancements in organic electronics. These orbitals are utilized in the design of organic semiconductors, organic light-emitting diodes (OLEDs), and organic solar cells.
Relationship with Hückel’s Rule
Pi molecular orbitals follow Hückel’s rule, which states that aromatic compounds must have a certain number of pi electrons to exhibit aromaticity. This rule helps predict and understand the behavior of pi systems in various organic molecules.
Pi Molecular Orbitals in Natural Products
Many natural products, such as flavonoids and polyaromatic hydrocarbons, feature pi molecular orbitals in their structures. These orbitals play a crucial role in determining their biological activities and properties.
Pi Molecular Orbitals in Transition Metal Complexes
Pi molecular orbitals are not limited to organic compounds; they also play a significant role in transition metal complexes. These orbitals can participate in metal-ligand bonding and influence the reactivity and stability of these complexes.
Computational Tools for Studying Pi Molecular Orbitals
Advancements in computational chemistry have provided powerful tools for studying pi molecular orbitals. Methods such as density functional theory (DFT) and molecular orbital calculations allow researchers to analyze and predict the behavior of these orbitals in various systems.
As we have seen, the 12 surprising facts about pi molecular orbitals demonstrate their crucial role in organic chemistry, aromaticity, electronic devices, and natural product chemistry. Understanding the behavior and properties of these orbitals unlocks new possibilities in various fields, from drug design to materials science.
Conclusion
In conclusion, the field of pi molecular orbitals is a fascinating area of study within chemistry. The unique properties and behaviors of pi molecular orbitals contribute to the understanding of chemical bonding, electronic structure, and reactivity. By delving into the surprising facts about pi molecular orbitals, we have gained insight into their significance in various chemical processes.From their role in aromaticity and conjugation to their impact on the electronic properties of molecules, pi molecular orbitals play a crucial role in shaping the behavior of organic compounds. Their distribution and arrangement greatly influence the spectroscopic properties of materials, making them valuable tools in characterizing compounds.Whether you are an aspiring chemist or simply curious about the wonders of nature, understanding pi molecular orbitals is essential to unraveling the complexities of chemical systems. So, next time you encounter the concept of pi molecular orbitals, remember these surprising facts and appreciate the vital role they play in the world of chemistry.
FAQs
1. What are pi molecular orbitals?
Pi molecular orbitals are molecular orbitals that result from the overlapping of p orbitals in a molecule. They are involved in the delocalized electron density above and below the plane of a molecule, often found in conjugated systems.2. What is the significance of pi molecular orbitals?
Pi molecular orbitals play a crucial role in determining the chemical reactivity, electronic properties, and spectroscopic behavior of organic compounds. They contribute to concepts such as aromaticity, conjugation, and delocalization of electrons.3. How are pi molecular orbitals different from sigma molecular orbitals?
Pi molecular orbitals result from the overlap of p orbitals perpendicular to the internuclear axis, while sigma molecular orbitals arise from the overlap of orbitals along the internuclear axis. Pi bonds are typically weaker than sigma bonds but confer unique properties to molecules.4. Can pi molecular orbitals exist in inorganic compounds?
Yes, pi molecular orbitals are not exclusive to organic compounds. They can also be present in inorganic compounds, especially those with conjugated systems or double bonds involving p orbitals.5. How do pi molecular orbitals contribute to aromaticity?
Pi molecular orbitals are responsible for aromaticity in organic compounds. Aromatic compounds have a fully conjugated pi system, leading to a stable, delocalized electron cloud that exhibits unique properties such as enhanced stability and distinctive chemical reactivity.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
