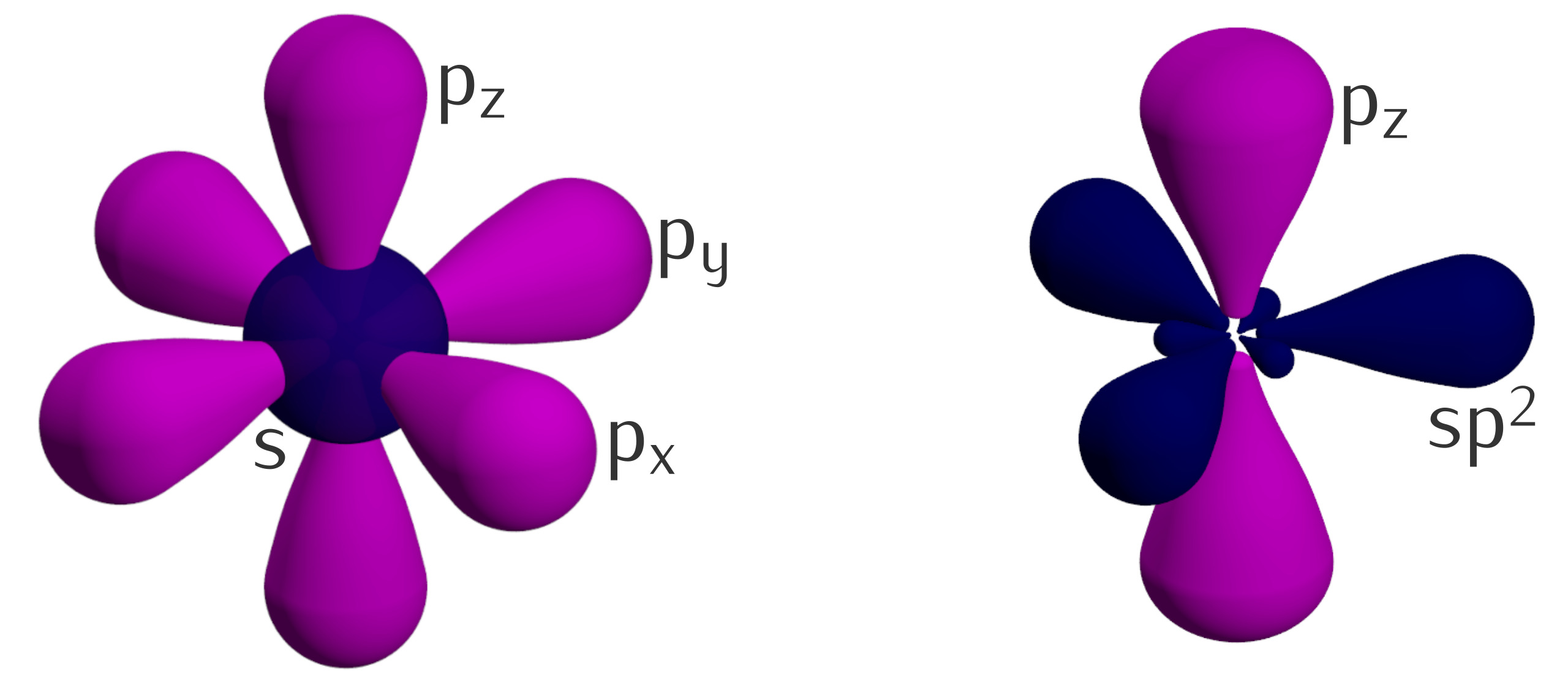
Hybrid orbitals are a fascinating concept in chemistry that play a crucial role in understanding the bonding and molecular structure of molecules. These hybrid orbitals are a combination of atomic orbitals that form when atoms bond together to form molecules. They help to explain the unique geometric arrangements of atoms in molecules and determine their chemical properties.
In this article, we will explore 16 unbelievable facts about hybrid orbitals that will deepen your understanding of this fundamental concept in chemistry. From the basics of hybridization to its applications in predicting molecular geometries and explaining the properties of molecules, these facts will demonstrate the importance and versatility of hybrid orbitals in the world of chemistry.
Key Takeaways:
- Hybrid orbitals are like special shapes that atoms make when they join together, and they help determine the shape and properties of molecules. They’re super important in understanding how things in the world around us are put together!
- By mixing and matching their orbitals, atoms create hybrid orbitals that allow them to form strong bonds and do cool things like make double and triple bonds. This helps create the amazing variety of substances we see in the world.
Hybrid orbitals are formed through the combination of atomic orbitals.
When different atomic orbitals mix together, hybrid orbitals are formed. This results in new orbitals with different shapes and energy levels, allowing for the bonding of atoms in a molecule.
Hybrid orbitals exhibit unique geometries.
Depending on the type of hybridization, hybrid orbitals can take on different shapes, such as sp, sp2, sp3, and sp3d. These geometries determine the molecular shape and the arrangement of atoms in a molecule.
Hybrid orbitals play a crucial role in determining molecular bond angles.
The hybridization of orbitals influences the angles between the bonds in a molecule. This is particularly important in understanding the structure and properties of organic compounds.
Hybrid orbitals allow for efficient overlapping of atomic orbitals.
By having hybrid orbitals, atoms in a molecule can effectively overlap their orbitals, leading to the formation of strong chemical bonds. This is essential for the stability and reactivity of compounds.
Hybrid orbitals are commonly found in carbon compounds.
Carbon, being a versatile element, readily forms hybrid orbitals to achieve different bonding arrangements. This is why carbon-based compounds, such as organic molecules, exhibit a wide range of structures and properties.
Hybrid orbitals enable the formation of double and triple bonds.
Through hybridization, atoms can form hybrid orbitals that have the ability to participate in multiple bond formations. This allows for the creation of double and triple bonds, which are crucial for the diversity of chemical reactions.
Hybrid orbitals explain the stability of certain molecules.
By optimizing the arrangement of electrons and minimizing electron repulsion, hybrid orbitals contribute to the stability of certain molecules, such as those with symmetric structures.
Valence bond theory is used to describe hybrid orbitals.
The concept of hybrid orbitals is closely associated with the valence bond theory, which explains the formation of chemical bonds through the overlap of atomic orbitals.
Hybridization can occur in elements beyond carbon.
Although carbon is the primary example of hybridization, other elements, such as nitrogen, oxygen, and sulfur, can also undergo hybridization to accommodate bonding requirements in different compounds.
Hybrid orbitals provide unique electronic configurations.
By combining atomic orbitals, hybrid orbitals offer distinct electronic configurations that contribute to the chemical reactivity and behavior of molecules.
Hybridization affects the pi bonding in molecules.
The type of hybrid orbitals involved in a molecule influences the presence and strength of pi bonding, which plays a crucial role in the stability and properties of conjugated systems.
The concept of hybrid orbitals helped revolutionize our understanding of chemical bonding.
When hybrid orbitals were introduced, they provided a new perspective on the nature of chemical bonds and helped explain many previously unexplained phenomena in chemistry.
Hybridization can result in the formation of delocalized electrons.
In certain compounds, hybrid orbitals can lead to the formation of delocalized electrons, offering unique electronic properties and contributing to the conductivity of materials like graphene.
Hybrid orbitals can influence the acidity and basicity of molecules.
The type of hybridization and the arrangement of atoms in a molecule can impact its acidity and basicity, influencing chemical reactions and the behavior of compounds in solution.
Hybrid orbitals are a cornerstone of organic chemistry.
In organic chemistry, hybridization is extensively used to explain the bonding and behavior of carbon-based compounds, which form the basis of life and are crucial in numerous industrial applications.
Hybrid orbital theory has practical applications in various scientific fields.
From understanding molecular structure and reactivity to designing new drugs and materials, hybrid orbital theory is widely used in fields such as pharmaceuticals, materials science, and molecular biology.
These 16 unbelievable facts about hybrid orbitals highlight the importance and versatility of this concept in the world of chemistry. Whether you are a student diving into the intricacies of molecular bonding or a curious individual interested in the wonders of the scientific world, exploring hybrid orbitals is sure to expand your knowledge and appreciation for the amazing complexities of the atomic realm.
Conclusion
In conclusion, hybrid orbitals are a fascinating concept in the world of chemistry. These unique molecular orbitals are formed when atomic orbitals mix together, resulting in a combination of characteristics that allow for greater flexibility and bonding capabilities. Hybrid orbitals play a crucial role in determining the geometry and properties of molecules, influencing everything from their shape to their reactivity.From the sp orbitals found in linear molecules to the sp2 and sp3 orbitals present in trigonal planar and tetrahedral molecules respectively, hybrid orbitals provide a deeper understanding of molecular structure and bonding. With hybridization, previously unexplained phenomena, such as the formation of double and triple bonds, can now be easily rationalized.By knowing more about hybrid orbitals, scientists can better understand the complexities of chemical reactions, molecular shapes, and the behavior of various compounds. Continued research in this area will undoubtedly uncover even more fascinating facts about hybrid orbitals, further expanding our knowledge of the intricate world of chemistry.
FAQs
Q: What are hybrid orbitals?
A: Hybrid orbitals are formed when atomic orbitals mix together, resulting in a combination of characteristics that allow for greater bonding capabilities and molecular geometry determination.
Q: How are hybrid orbitals classified?
A: Hybrid orbitals are classified based on the type of mixing involved. Some common hybrid orbitals include sp, sp2, and sp3 orbitals.
Q: What is the significance of hybrid orbitals?
A: Hybrid orbitals are significant because they provide a deeper understanding of molecular structure, bonding, and the behavior of various compounds.
Q: How do hybrid orbitals influence molecular shape?
A: Hybrid orbitals determine molecular shape by influencing the arrangement of atoms and bonds around the central atom.
Q: Can you give examples of molecules with hybrid orbitals?
A: Examples of molecules with hybrid orbitals include methane (CH4) with sp3 hybridization, ethene (C2H4) with sp2 hybridization, and ethyne (C2H2) with sp hybridization.
Q: How do hybrid orbitals explain the formation of double and triple bonds?
A: Hybrid orbitals explain the formation of double and triple bonds by allowing for the overlap of orbitals and the sharing of electrons in these types of bonds.
Q: What are some future research areas related to hybrid orbitals?
A: Future research areas related to hybrid orbitals could include further exploring different types of hybridization, investigating the effects of hybridization on molecular reactivity, and exploring the role of hybrid orbitals in complex molecular systems.
Hybrid orbitals are truly remarkable, but there's still more to explore! If you're curious about how atomic orbitals combine and rearrange, our article on orbital hybridization will blow your mind. Unravel the secrets behind molecular geometry and bonding, and prepare to be amazed by the fascinating world of chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
