Welcome to the fascinating world of atomic orbitals! In the vast realm of physics, atomic orbitals play a crucial role in understanding the behavior of electrons within an atom. These invisible regions of space around the nucleus hold the key to unraveling the mysteries of electronic structure and the properties of elements. From the earliest theories proposed by Niels Bohr to the complex quantum mechanical models of today, our understanding of atomic orbitals has evolved and continues to expand. In this article, we will delve into 19 astounding facts about atomic orbitals that will leave you in awe of the intricate nature of the atomic world. So, fasten your seatbelts and get ready to embark on an exhilarating journey through the realm of atomic orbitals!
Key Takeaways:
- Atomic orbitals are regions where electrons hang out in atoms. They have different shapes and energy levels, and they determine how elements behave in chemistry. It’s like a secret dance party for electrons!
- Understanding atomic orbitals helps scientists explain how atoms bond and form molecules. It’s like knowing the secret recipe for creating all the different kinds of matter in the universe!
Atomic orbitals are regions of space where electrons are most likely to be found.
The concept of atomic orbitals revolutionized our understanding of how electrons behave within an atom. They provide a visual representation of the probability distribution of finding an electron at a specific location.
Electrons occupy atomic orbitals in specific energy levels.
Each energy level has a maximum number of orbitals it can hold, with the innermost levels having fewer orbitals than the outer ones.
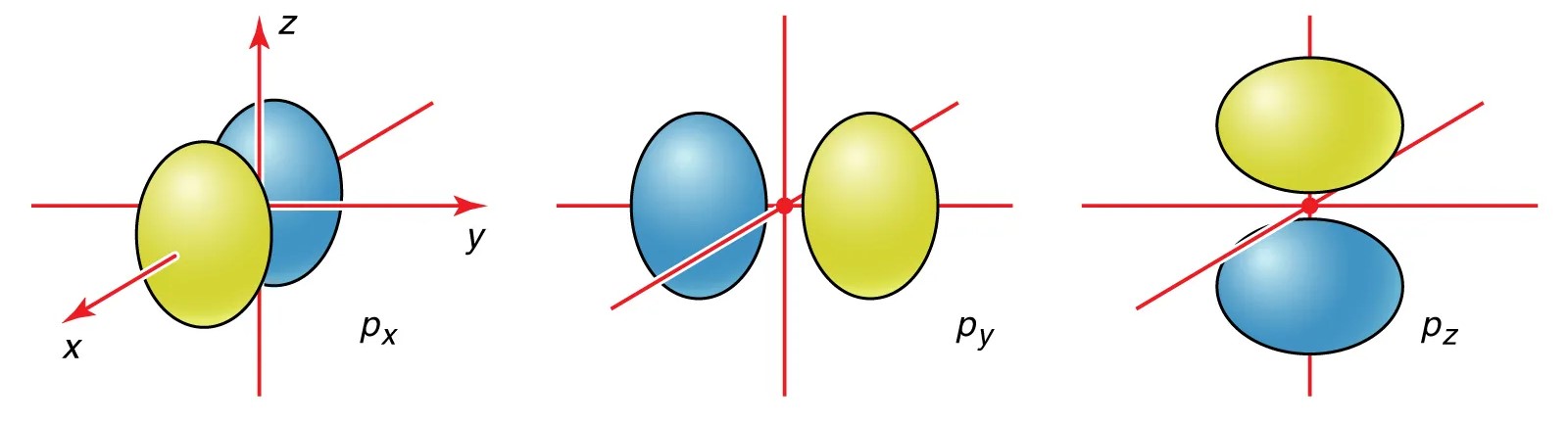
Atomic orbitals have distinct shapes.
There are different types of atomic orbitals, denoted by letters such as s, p, d, and f. Each of these orbitals has a characteristic shape and orientation within the atom.
The s orbital is spherical in shape.
The s orbital is the simplest and has a spherical shape. It is closest to the nucleus and can hold up to two electrons.
The p orbital is dumbbell-shaped.
The p orbital has two lobes, with a node in the center. It can hold up to six electrons, distributed among the three p orbitals.
Atomic orbitals have different energy levels.
The energy of an orbital depends on its distance from the nucleus. Orbitals closer to the nucleus have lower energy levels.
Orbitals can overlap.
When orbitals belonging to different atoms overlap, chemical bonding occurs. The extent of orbital overlap determines the strength of the bond.
Electron spin is an intrinsic property of atomic orbitals.
Electrons in an atomic orbital possess a property called spin, which can be either up or down. This property helps in understanding electron behavior within an atom.
Quantum mechanics describes atomic orbitals.
The behavior of electrons in atomic orbitals is described by quantum mechanics, a branch of physics that deals with particles at the atomic and subatomic levels.
Atomic orbitals follow the Pauli exclusion principle.
The Pauli exclusion principle states that no two electrons within an atom can have the same set of quantum numbers. This principle explains why there are a limited number of electrons in each orbital.
Atomic orbitals determine the chemical properties of elements.
The arrangement and filling of atomic orbitals determine the chemical behavior of elements, including their reactivity and ability to form compounds.
Atomic orbitals are quantized.
Electrons can only occupy specific energy levels and orbitals within an atom. This quantization of energy levels is a fundamental aspect of atomic structure.
Atomic orbitals can be visualized using electron cloud models.
Electron cloud models provide a way to visualize atomic orbitals. They depict the probability distribution of finding an electron in a particular region of space.
The shape of atomic orbitals affects molecular geometry.
The arrangement and orientation of atomic orbitals influence the shape and structure of molecules. This, in turn, determines their physical and chemical properties.
Atomic orbitals can be combined to form hybrid orbitals.
Hybrid orbitals are a combination of atomic orbitals, resulting in new orbitals of different shapes and orientations. These hybrid orbitals are involved in the bonding of atoms in molecules.
The number of atomic orbitals increases with energy level.
As we move to higher energy levels, the number of atomic orbitals available increases. This allows for more complex arrangements of electrons within an atom.
The Aufbau principle governs the filling of atomic orbitals.
The Aufbau principle states that electrons occupy the lowest energy orbitals available first before filling higher energy levels. This principle helps in determining electron configurations.
Atomic orbitals can have degenerate energy levels.
Degenerate orbitals have the same energy level but differ in spatial orientation. They play a crucial role in determining the electronic structure of atoms.
Atomic orbitals provide a foundation for understanding chemical bonding.
By understanding atomic orbitals, scientists can explain the types of chemical bonds that form between atoms. These bonds are responsible for the formation of molecules and the diversity of matter.
These 19 astounding facts about atomic orbitals shed light on the fascinating world of subatomic particles and their behavior within an atom. Atomic orbitals play a crucial role in determining the properties and reactivity of elements, paving the way for groundbreaking discoveries in chemistry and quantum physics. Whether visualizing the electron cloud or understanding electron spin, the study of atomic orbitals continues to captivate scientists and deepen our understanding of the fundamental building blocks of the universe.
Conclusion
In conclusion, atomic orbitals play a crucial role in understanding the behavior and properties of atoms. These fascinating regions of space around the nucleus provide a framework for the arrangement of electrons. Through the study of atomic orbitals, scientists have been able to uncover the intricate details of atomic structure and the periodic table.From the different shapes and orientations of orbitals to the principles governing their filling, atomic orbitals have contributed significantly to our comprehension of chemistry and physics. This knowledge has led to advancements in various fields, such as materials science, nanotechnology, and quantum mechanics.As we continue to delve deeper into the world of atomic orbitals, we uncover even more astonishing facts and applications. By embracing these fundamental concepts, we gain a better understanding of the building blocks of matter and unlock the potential for groundbreaking discoveries in the future.
FAQs
1. What is an atomic orbital?
An atomic orbital is a mathematical function that describes the probability distribution of finding an electron in a specific region around an atom’s nucleus.
2. How are atomic orbitals classified?
Atomic orbitals are classified based on their shape, which can be spherically symmetric (s orbitals), dumbbell-shaped (p orbitals), or more complex (d and f orbitals).
3. How do electrons fill atomic orbitals?
Electrons follow the Aufbau principle, which states that they fill the lowest energy orbitals first before moving to higher energy orbitals according to the Pauli exclusion principle and Hund’s rule.
4. Can an atomic orbital contain more than two electrons?
Yes, atomic orbitals can contain up to a maximum of two electrons. These electrons must have opposite spins as dictated by the Pauli exclusion principle.
5. Do all atoms have the same number of atomic orbitals?
No, the number of atomic orbitals depends on the element and its atomic number. Elements with higher atomic numbers have more orbitals due to the increasing numbers of electrons.
6. What are hybrid orbitals?
Hybrid orbitals are a combination of atomic orbitals that result from the mixing of orbitals to form new orbitals. They play a significant role in explaining molecular geometry in compounds.
7. Can atomic orbitals overlap?
Yes, atomic orbitals can overlap, leading to the formation of molecular orbitals in chemical compounds. These molecular orbitals allow for the sharing of electrons between atoms.
8. Are atomic orbitals visible?
No, atomic orbitals are not visible in the traditional sense. They are mathematical constructs used to describe electron behavior and are represented through models and diagrams.
9. How do atomic orbitals contribute to chemical bonding?
By overlapping and sharing electrons, atomic orbitals contribute to the formation of chemical bonds. This allows atoms to combine and create stable compounds.
10. Are atomic orbitals static or dynamic?
Atomic orbitals are constantly in motion due to the inherent uncertainty associated with the behavior of electrons. Their exact position and momentum cannot be simultaneously determined.
Atomic orbitals provide a captivating glimpse into the quantum world of electrons. Delving deeper into this fascinating subject, you can explore the intricacies of electron configuration with Hund's rule, which governs the filling of orbitals with parallel spins. Hybrid orbitals, formed by the combination of atomic orbitals, offer a unique perspective on molecular geometry and bonding. Lastly, sigma bonds, the strongest type of covalent bond, play a crucial role in holding molecules together. Continue your journey through the quantum realm and uncover more mind-boggling facts about these essential concepts in chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
