The ground state is a fascinating concept in the field of chemistry that refers to the lowest energy level of an atom or molecule. It is a state of stability where electrons occupy their lowest energy orbitals. Understanding the ground state is crucial in comprehending the behavior and properties of matter.
In this article, we will explore 14 astonishing facts about the ground state that will shed light on its significance in the world of chemistry. From the arrangement of electrons to the impact on chemical reactivity, we will delve into the intriguing details of this fundamental concept. So, let’s dive in and unlock the secrets of the ground state!
Key Takeaways:
- The ground state is like the cozy, stable home for electrons in atoms and molecules, keeping them in their lowest energy levels for maximum stability and minimal repulsion.
- Understanding the ground state helps scientists predict chemical reactions and explain why elements behave the way they do, like a secret code that unlocks the mysteries of chemistry.
Ground State: The Foundation of Chemistry
The ground state is a fundamental concept in chemistry, serving as the starting point for understanding the behavior of atoms and molecules. It is the lowest energy state that an electron can occupy within an atom or molecule.
Striving for Stability
Atoms and molecules naturally tend to be in their ground state, as it represents the most stable configuration. In this state, electrons are arranged in the lowest energy levels, ensuring minimal energy and maximum stability.
Quantum Mechanical Description
The ground state is described by quantum mechanics, a branch of physics that deals with the behavior and properties of particles on the atomic and subatomic scale. Quantum mechanics provides a mathematical framework for understanding the distribution of electrons in atoms and molecules.
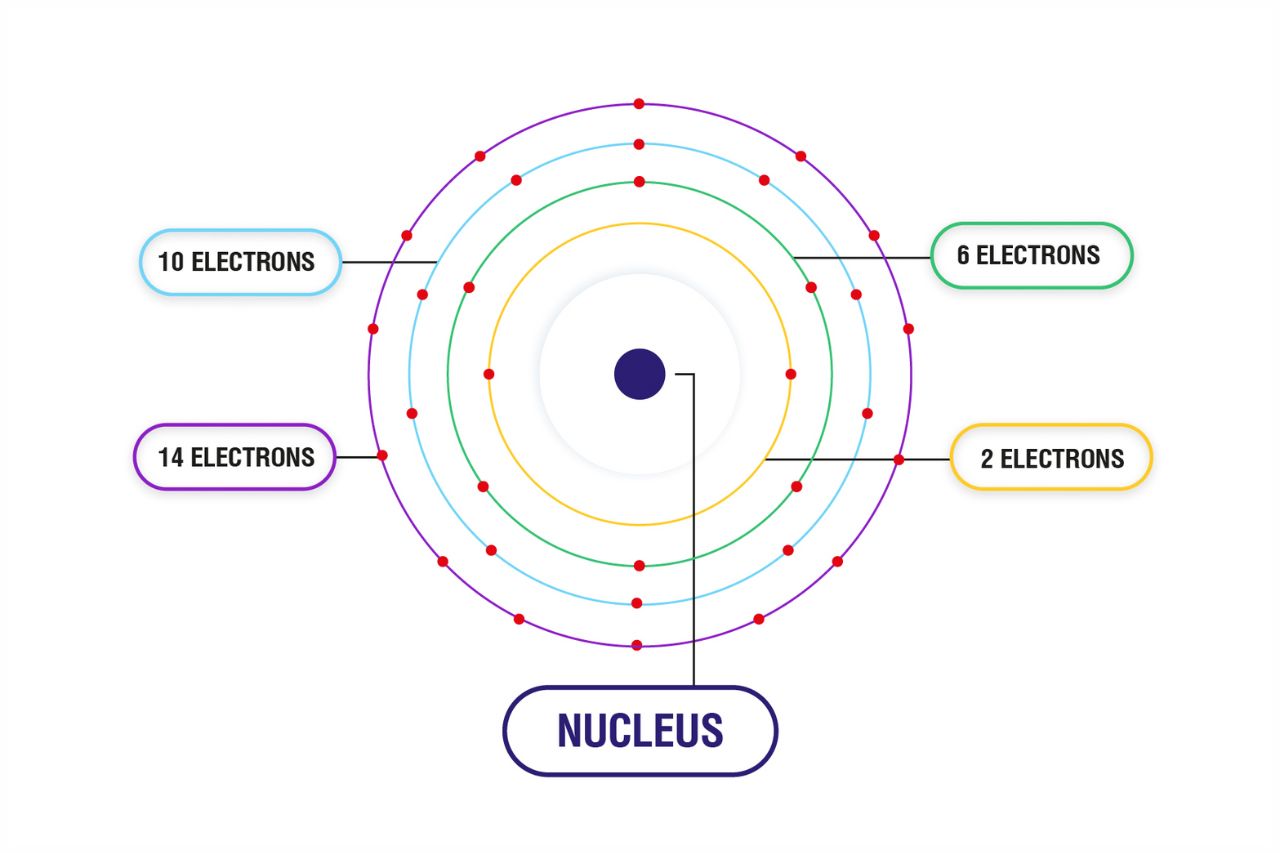
Electronic Configuration
An atom’s ground state is determined by the arrangement of its electrons in different energy levels or orbitals. The electronic configuration of an atom specifies how many electrons are present in each orbital, following specific rules based on quantum mechanics.
Lower Energy, Higher Stability
In the ground state, electrons occupy the lowest energy levels available. This configuration minimizes the repulsion between electrons and maximizes the attraction between electrons and the nucleus, resulting in a stable arrangement.
Absorption and Emission of Energy
When energy is absorbed by an atom, an electron can be excited to a higher energy level. However, the excited electron will eventually return to the ground state by releasing the absorbed energy in the form of light or heat, a process known as emission.
Relationship to Chemical Bonding
The ground state plays a crucial role in chemical bonding. Atoms form chemical bonds by sharing or transferring electrons to achieve a stable electron configuration similar to the ground state.
The Role of Valence Electrons
In chemical reactions, valence electrons are primarily involved. These are the electrons in the outermost energy levels of an atom, responsible for determining an element’s chemical properties and reactivity.
Ground State and Electron Configuration Notation
Ground state electron configurations are often represented using the electron configuration notation, which shows the distribution of electrons in different orbitals using letters and numbers.
Energy Levels and Sublevels
An atom’s electrons are organized into energy levels and sublevels. The energy levels, designated by principal quantum numbers (n), are further divided into sublevels (s, p, d, f) to describe different orbitals with specific shapes and orientations.
Ground State and the Aufbau Principle
The Aufbau principle states that electrons fill the lowest energy orbitals first before occupying higher energy levels. This principle governs the arrangement of electrons in atoms and helps determine the ground state configuration.
Influence on Spectroscopy
Spectroscopy, the study of the interaction of matter with light, heavily relies on understanding the ground state and electronic transitions. By studying how atoms and molecules absorb and emit light at specific energies, scientists gain valuable information about their electronic structure.
Excited States and Ground State Transitions
Excited states occur when electrons are in higher energy levels. The transition from an excited state to the ground state results in the emission of energy in the form of photons, which can be detected and analyzed in spectroscopic experiments.
Significant Impact on Chemical Properties
The ground state and its corresponding electron configuration greatly influence an element’s chemical properties and behavior. Elements in the same group or period have similar ground state configurations, leading to similar reactions and bonding tendencies.
Overall, the ground state is a fundamental concept in chemistry that underpins many aspects of atomic and molecular behavior. Understanding the ground state and its implications allows scientists to make predictions, explain properties, and explore the fascinating world of chemistry.
Conclusion
The ground state is a fascinating concept in the world of chemistry. It represents the lowest energy level that an atom or molecule can occupy. Understanding the ground state is essential for understanding the behavior and properties of different chemical species. Through this article, we have explored 14 astonishing facts about the ground state, including its significance in the stability of matter, the role of electron configuration, and the impact on chemical reactivity.
Overall, the ground state plays a crucial role in determining the behavior of atoms and molecules, and studying it has revolutionized our understanding of the chemical world. By delving into the intricacies of the ground state, scientists and researchers have been able to unlock countless insights and applications across various fields, from materials science to drug discovery. The study of the ground state continues to be a vibrant area of research, promising further discoveries and advancements in the future.
FAQs
Q: What is the ground state?
A: The ground state is the lowest energy level that an atom or molecule can occupy. It represents the most stable configuration of electrons within an atom or molecule.
Q: Why is the ground state important?
A: The ground state is important because it influences the behavior and properties of atoms and molecules. It determines their reactivity, stability, and overall chemical characteristics.
Q: How is the ground state determined?
A: The ground state is determined by the arrangement of electrons around the nucleus of an atom or molecule. It follows certain rules and principles, such as the Pauli exclusion principle and Hund’s rule.
Q: Can the ground state change?
A: The ground state can change under specific conditions, such as when an external energy source is applied to an atom or molecule. This can cause electrons to move to higher energy levels, resulting in a new ground state.
Q: How does the ground state affect chemical reactions?
A: The ground state influences the energy available for chemical reactions. It determines the stability of atoms and molecules and affects their ability to interact with other species, leading to the formation or breaking of chemical bonds.
Q: What are excited states?
A: Excited states are higher energy levels that atoms or molecules can occupy when given additional energy. These states are temporary and unstable, with electrons occupying higher orbitals than in the ground state.
Q: Can the ground state be calculated?
A: Yes, the ground state can be calculated using quantum mechanical methods, such as Hartree-Fock theory or density functional theory. These calculations require complex mathematical equations to obtain the electronic structure of a system in its ground state.
Q: Is the ground state the same for all elements?
A: No, the ground state varies from element to element. Each element has its unique electron configuration and energy levels, resulting in different ground state configurations.
Q: How do chemists experimentally determine the ground state?
A: Chemists use various techniques, such as spectroscopy and electron energy loss spectroscopy, to probe the electronic structure and energy levels of atoms and molecules. These experiments provide insights into the ground state configuration.
Q: Can the ground state be observed directly?
A: The ground state itself cannot be directly observed because it represents the lowest energy level. However, its effects and transitions between different energy states can be observed through spectroscopic techniques.
Q: Are there different ground states for isotopes of the same element?
A: Isotopes of the same element have the same electron configuration, and thus the same ground state. However, the overall energy of the ground state may differ slightly due to the variations in nuclear mass.
Q: Can changing the ground state of an atom or molecule have practical applications?
A: Yes, changing the ground state of an atom or molecule can have practical applications. It can lead to the development of new materials, catalysts, and electronic devices, as well as advances in fields like quantum computing.
Q: Are there any exceptions to the rules governing the ground state?
A: While the rules governing the ground state are generally reliable, there are exceptions, especially for transition metals and heavier elements. These exceptions arise due to factors like electron-electron interactions and relativistic effects.
Q: What role does quantum mechanics play in understanding the ground state?
A: Quantum mechanics is essential for understanding the ground state. It provides the theoretical framework to describe the behavior of electrons, their energy levels, and their distribution around the nucleus, which all contribute to defining the ground state of an atom or molecule.
Ground state is just the beginning of the quantum world's wonders. Delving deeper into atomic energy levels reveals the captivating realm of excited states, where electrons jump to higher orbitals. Beyond the subatomic domain, nature's marvels await at the renowned San Diego Zoo, home to countless amazing creatures. Chemistry's colorful side comes alive through the mesmerizing flame test, which unveils the unique spectral fingerprints of elements. Explore these enthralling topics and expand your knowledge of the incredible world around us.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
