Molecular Orbital Theory is a fundamental concept in the field of Chemistry that helps us understand the behavior and properties of molecules. It provides valuable insights into the bonding and electronic structure of molecules, allowing us to predict their stability, reactivity, and spectroscopic properties. In this article, we will explore 17 astounding facts about Molecular Orbital Theory that will deepen your understanding of this fascinating subject.From the simple concept of electron orbitals to the complex diagrams that represent molecular orbitals, Molecular Orbital Theory has revolutionized the way we comprehend chemical bonding. Whether you’re a student studying Chemistry or simply curious about the intricacies of molecules, these facts will amaze and engage you. So, let’s dive in and uncover the wonders of Molecular Orbital Theory!
Key Takeaways:
- Molecular Orbital Theory explains how atoms bond to form molecules, revealing the secrets of electron interactions and energy levels within substances. It’s like a molecular puzzle that unlocks the mysteries of chemistry!
- Molecular Orbital Theory predicts molecule stability, shapes, and even magnetic properties. It’s like a superpower for chemists, helping them design drugs and understand the building blocks of matter.
Molecular Orbital Theory Explains Chemical Bonding
Molecular Orbital Theory is a fundamental concept in chemistry that describes how chemical bonding occurs between atoms to form molecules. It provides a deeper understanding of the interactions between electrons and the energy levels within molecules.
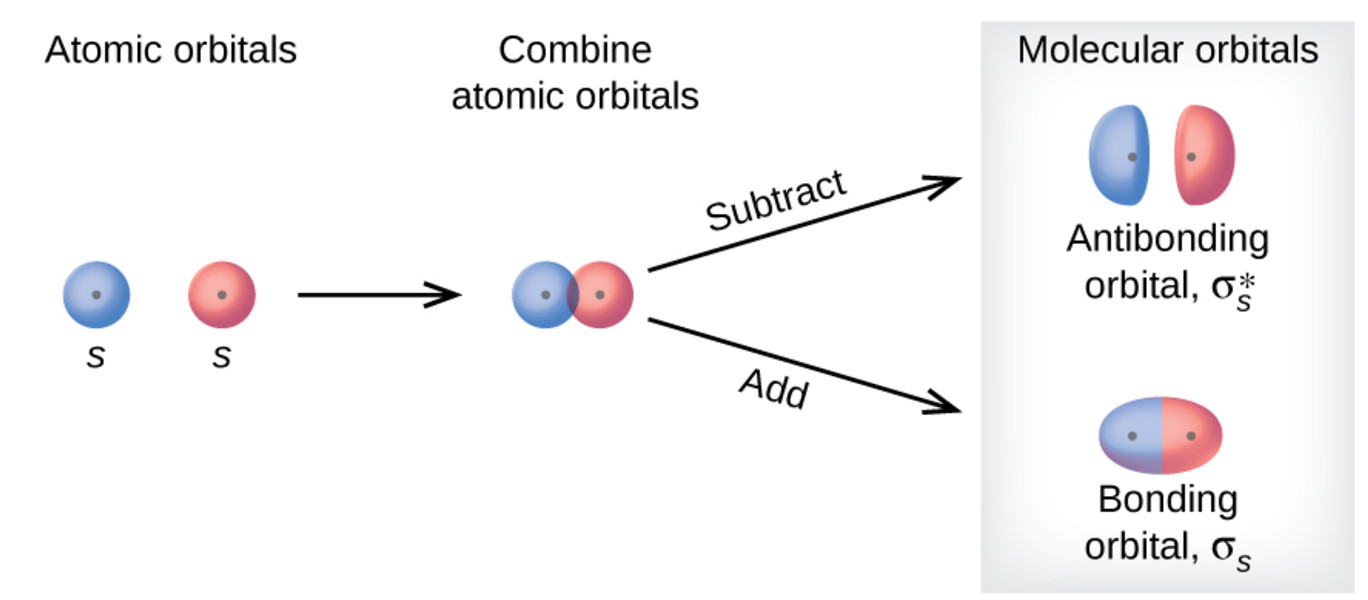
The Key Idea: Combining Atomic Orbitals
The central idea in Molecular Orbital Theory is to combine atomic orbitals from different atoms to form molecular orbitals. These molecular orbitals can hold a maximum of two electrons, following the rules of electron filling and the Pauli exclusion principle.
Two Types of Molecular Orbitals
In Molecular Orbital Theory, there are two types of molecular orbitals: bonding and anti-bonding orbitals. Bonding orbitals result from constructive interference, where the overlapping atomic orbitals reinforce each other, leading to increased electron density between the nuclei. Anti-bonding orbitals, on the other hand, result from destructive interference and have a node between the nuclei.
The Interaction Diagram
The interaction between atomic orbitals to form molecular orbitals can be represented graphically using an interaction diagram. This diagram shows the energy levels of the atomic orbitals and the resulting molecular orbitals, indicating which orbitals are bonding and anti-bonding.
Molecular Orbitals Determine Molecule Stability
The stability of a molecule is determined by the population of its molecular orbitals. If the bonding orbitals are more populated than the anti-bonding orbitals, the molecule is stable. However, if the anti-bonding orbitals are more populated, the molecule is less stable and more likely to undergo chemical reactions.
The Bond Order
The bond order is a measure of the strength of the bond between two atoms in a molecule. It is calculated by subtracting the number of anti-bonding electrons from the number of bonding electrons and dividing by two. A higher bond order indicates a stronger bond.
The Hydrogen Molecule Orbitals
The simplest example of Molecular Orbital Theory is the hydrogen molecule. When two hydrogen atoms come together, their atomic orbitals combine to form a bonding orbital (?) and an anti-bonding orbital (?*). The bonding orbital is lower in energy, making the hydrogen molecule stable.
Orbital Overlap and Bond Strength
The strength of a chemical bond is directly related to the extent of overlap between the atomic orbitals involved in bonding. Greater overlap leads to stronger bonds due to increased electron density between the nuclei.
Explanation for Molecular Shapes
Molecular Orbital Theory provides an explanation for molecular shapes. The shape of a molecule is determined by the spatial distribution of its molecular orbitals and the electron density between the nuclei.
Hybridization and Molecular Orbitals
Hybridization, a concept in Molecular Orbital Theory, explains the mixing of atomic orbitals to form hybrid orbitals. These hybrid orbitals then combine with other atomic or hybrid orbitals to form molecular orbitals.
Spectroscopic Techniques and Molecular Orbitals
Molecular Orbital Theory is utilized in various spectroscopic techniques to determine molecular structures and analyze electronic transitions. Spectroscopy provides valuable information about the energy levels and bonding in molecules.
Electron Delocalization in Conjugated Systems
Molecular Orbital Theory explains electron delocalization in conjugated systems, such as in aromatic compounds like benzene. The delocalized pi (?) molecular orbitals give rise to unique properties, including enhanced stability and unique reactivity.
Molecules with Multiple Bonds
In Molecular Orbital Theory, molecules with multiple bonds, like double or triple bonds, can be explained by the concept of overlapping atomic orbitals forming different types of molecular orbitals.
Prediction of Magnetic Properties
Molecular Orbital Theory can predict the magnetic properties of molecules. Paramagnetic molecules have unpaired electrons in their molecular orbitals, causing them to be attracted to a magnetic field, whereas diamagnetic molecules have all their electrons paired.
Application in Drug Design
Molecular Orbital Theory plays a crucial role in drug design and computational chemistry. Understanding the electronic structure and interactions within molecules helps in predicting their biological activity and designing effective drugs.
Limitations and Approximations
Like any scientific theory, Molecular Orbital Theory has its limitations and approximations. It assumes the independent motion of electrons and neglects factors like electron-electron repulsion and electron correlation, which are important in more complex systems.
Quantum Mechanics and Molecular Orbitals
Molecular Orbital Theory is based on the principles of quantum mechanics. It provides a mathematical framework for understanding the behavior of electrons and their energy levels within molecules, allowing for accurate predictions of their properties and interactions.
Conclusion
Molecular orbital theory is a fascinating and powerful concept in chemistry that helps us understand the behavior and properties of molecules. Through the combination of atomic orbitals, we can create new molecular orbitals that result in stable and unique structures. This theory provides a deeper insight into the nature of chemical bonds, allowing scientists to predict and explain various phenomena.
By understanding molecular orbital theory, we have a better understanding of chemical reactions, molecular properties, and spectroscopic techniques. This knowledge has crucial applications in fields such as pharmaceuticals, materials science, and environmental studies. The 17 astounding facts presented in this article showcase the complexity and beauty of molecular orbital theory, highlighting the importance of this concept in the world of chemistry.
Overall, molecular orbital theory is an invaluable tool that unlocks the mysteries of molecular behavior, enabling scientists to push the boundaries of scientific discovery and innovation.
FAQs
Q: What is molecular orbital theory?
A: Molecular orbital theory is a concept in chemistry that describes the behavior and properties of molecules through the combination of atomic orbitals.
Q: How does molecular orbital theory explain chemical bonding?
A: Molecular orbital theory explains chemical bonding by forming new molecular orbitals through the combination of atomic orbitals, resulting in stable molecular structures.
Q: What are the applications of molecular orbital theory?
A: Molecular orbital theory has applications in various fields, including pharmaceuticals, materials science, and environmental studies, enabling scientists to understand chemical reactions, molecular properties, and spectroscopic techniques.
Q: Why is molecular orbital theory important?
A: Molecular orbital theory is important because it provides a deeper insight into the nature of chemical bonds and helps predict and explain various phenomena in the molecular world.
Q: Can molecular orbital theory be applied to all molecules?
A: Yes, molecular orbital theory can be applied to all molecules, regardless of their complexity. It provides a framework for understanding their behavior and properties.
Q: How does molecular orbital theory contribute to scientific innovation?
A: Molecular orbital theory contributes to scientific innovation by uncovering the mysteries of molecular behavior, enabling scientists to make new discoveries, develop novel materials, and design better drugs.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
