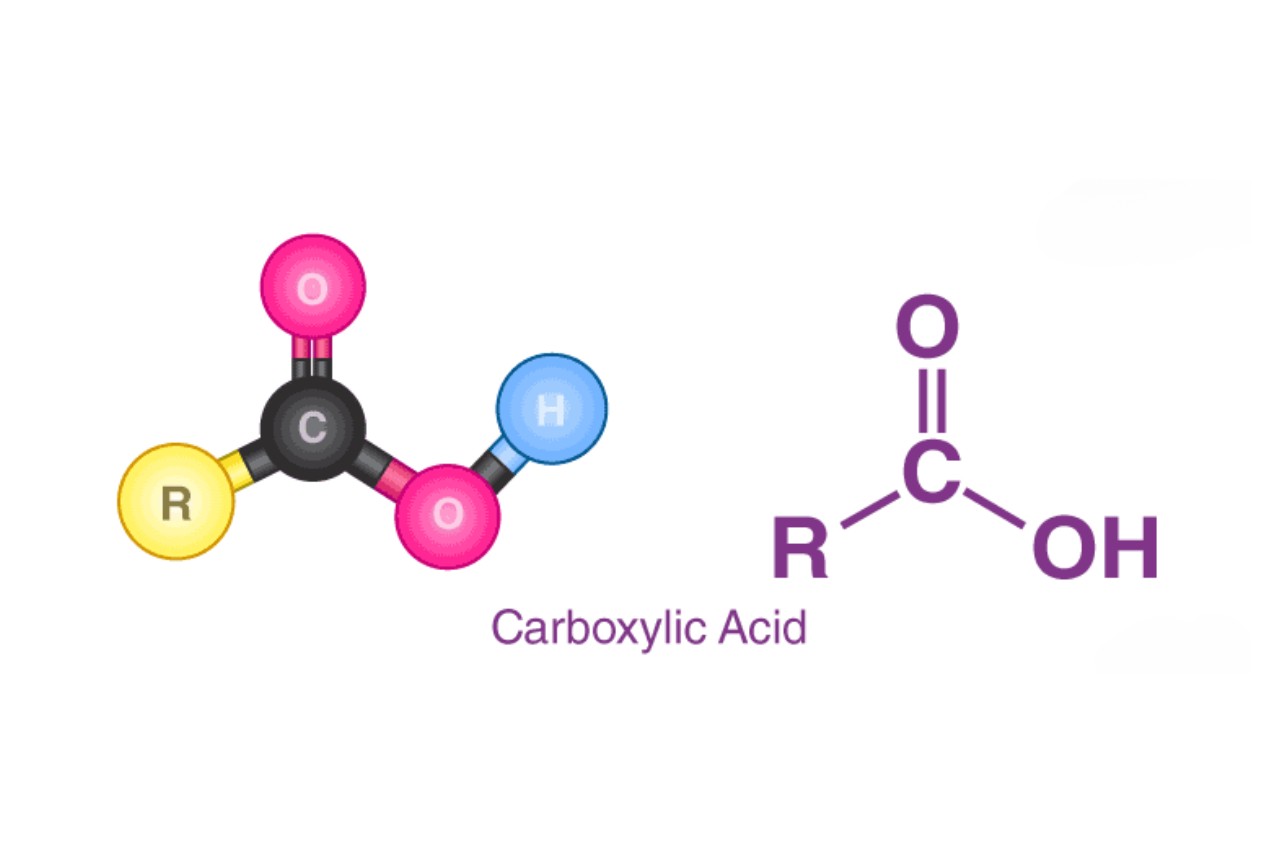
Carboxylic acids are a fascinating class of compounds found in various natural and synthetic substances. They are characterized by the presence of a carboxyl group (-COOH) in their molecular structure. These compounds play a vital role in many organic and biological processes, making them an essential topic to explore in the field of chemistry.
In this article, we will delve into some astounding facts about carboxylic acids that will deepen our understanding and appreciation for these compounds. From their diverse applications in industry and everyday life to their unique chemical properties, carboxylic acids prove to be truly remarkable substances. So, without further ado, let us embark on a journey into the intriguing world of carboxylic acids and unravel some of their secrets!
Key Takeaways:
- Carboxylic acids are versatile compounds found in nature and play a crucial role in biochemical processes, making them essential in various industries and organic synthesis.
- These acids have distinct physical properties, can be synthesized through different methods, and form salts and esters with various applications in food, fragrance, and organic chemistry.
Carboxylic acid is a versatile organic compound.
Carboxylic acids are a class of compounds that contain a carboxyl group (-COOH). This functional group gives them a wide range of applications in various industries, including pharmaceuticals, food and beverages, and polymers.
Carboxylic acids are found in numerous natural sources.
These acids can be obtained from both plant and animal sources. For example, acetic acid is found in vinegar, while citric acid is present in citrus fruits. Formic acid, on the other hand, is naturally secreted by ants.
Carboxylic acids play a crucial role in biochemical processes.
They are involved in many biochemical reactions, such as the Krebs cycle and fatty acid metabolism. Without carboxylic acids, these essential processes would not occur, impacting the overall functioning of living organisms.
Carboxylic acids have distinct physical properties.
These compounds are typically high boiling liquids or solids at room temperature. They have a sharp, pungent odor and can be corrosive in their concentrated form.
Carboxylic acids can be synthesized through various methods.
One common method is the oxidation of primary alcohols or aldehydes. Additionally, carboxylic acids can be obtained through the hydrolysis of esters or nitriles.
Carboxylic acids can form salts and esters.
When carboxylic acids react with bases, they form salts known as carboxylates. These salts have various applications, such as being used as food additives or preservatives. Carboxylic acids can also undergo esterification reactions to form esters, which are commonly used in the fragrance and flavor industry.
Carboxylic acids exhibit different levels of acidity.
The acidity of carboxylic acids can vary depending on the substituents attached to the carboxyl group. Factors such as electron-withdrawing or electron-donating groups can affect the stability of the carboxylate ion, thereby influencing the acidity of the compound.
Carboxylic acids are essential building blocks in organic synthesis.
These acids are extensively used in the production of various organic compounds, such as pharmaceuticals, dyes, and plastics. They serve as starting materials for many chemical reactions, making them indispensable in the field of organic chemistry.
Conclusion
In conclusion, carboxylic acids are fascinating compounds with a wide range of applications and unique properties. From their role in biochemical processes to their use in various industries, carboxylic acids play a crucial role in our everyday lives. We have explored eight astounding facts about carboxylic acids, highlighting their importance and significance in the field of chemistry. Whether you are a chemistry enthusiast or simply curious about the world around you, learning about carboxylic acids can deepen your understanding of the chemical world. Remember to always handle carboxylic acids with caution due to their corrosive nature and consult with experts when working with these compounds.
FAQs
1. What are carboxylic acids?
Carboxylic acids are organic compounds that contain a carboxyl group (COOH) attached to a carbon atom. They are characterized by their acidic properties.
2. What are some examples of carboxylic acids?
Common examples of carboxylic acids include acetic acid (found in vinegar), formic acid (found in ants), citric acid (found in citrus fruits), and butyric acid (found in butter).
3. What are the uses of carboxylic acids?
Carboxylic acids have various applications. They are used in the production of pharmaceuticals, food additives, solvents, and fragrances. They also play a crucial role in biochemical processes within our bodies.
4. Are carboxylic acids dangerous?
Some carboxylic acids can be corrosive and toxic. It is important to handle them with caution, wearing appropriate protective gear and following safety guidelines when working with these compounds.
5. Can carboxylic acids react with other compounds?
Yes, carboxylic acids can undergo various reactions, such as esterification, amidation, and decarboxylation, among others. These reactions are important in the synthesis of many organic compounds.
6. Are carboxylic acids naturally occurring?
Yes, carboxylic acids are commonly found in nature. They are produced by many living organisms and can be found in foods, plants, and even animals.
7. Can carboxylic acids be used as preservatives?
Indeed, certain carboxylic acids, such as benzoic acid and sorbic acid, are commonly used as preservatives in food and cosmetic products to prevent microbial growth.
8. What is the chemical structure of a carboxylic acid?
A carboxylic acid has a general chemical formula of RCOOH, where R represents an organic group. The carboxyl group consists of a carbonyl group (C=O) and a hydroxyl group (OH) attached to the same carbon atom.
Carboxylic acids' fascinating properties and applications make them essential in organic chemistry. Delving deeper into the world of functional groups, amides also possess surprising characteristics that captivate scientists and researchers alike. Exploring the unique features of amides and functional groups can lead to a greater understanding of their roles in various fields, from pharmaceuticals to materials science.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
