Chemical formulas are the building blocks of understanding the composition and behavior of different substances. They provide a concise representation of the elements present in a compound and the ratio in which they combine. While they may seem like a jumble of symbols and numbers to the untrained eye, chemical formulas hold a wealth of information waiting to be unlocked. In this article, we will explore 19 unbelievable facts about chemical formulas that will enhance your understanding of the fascinating world of chemistry. From the simple to the complex, these facts will showcase the importance and versatility of chemical formulas in a variety of applications. So, hold on tight as we dive deep into the intriguing world of chemical formula and discover some truly mind-blowing facts.
Key Takeaways:
- Chemical formulas are like the secret code of chemistry, helping scientists understand and communicate the building blocks of substances through symbols and numbers.
- By using chemical formulas, scientists can predict reactions, name compounds, ensure safety, and unlock the mysteries of molecular structures and properties.
Chemical formulas are the language of chemistry
Chemical formulas are like the vocabulary of chemistry, allowing scientists to communicate and represent the composition of substances using symbols and numbers.
Chemical formulas consist of elements and subscripts
A chemical formula is made up of elemental symbols that represent different types of atoms, such as H for hydrogen and O for oxygen, combined with subscripts that indicate the number of atoms of each element in a molecule.
Chemical formulas provide information on molecular structure
By studying the chemical formula of a compound, scientists can determine the arrangement of atoms within the molecule, providing insights into its properties and behavior.
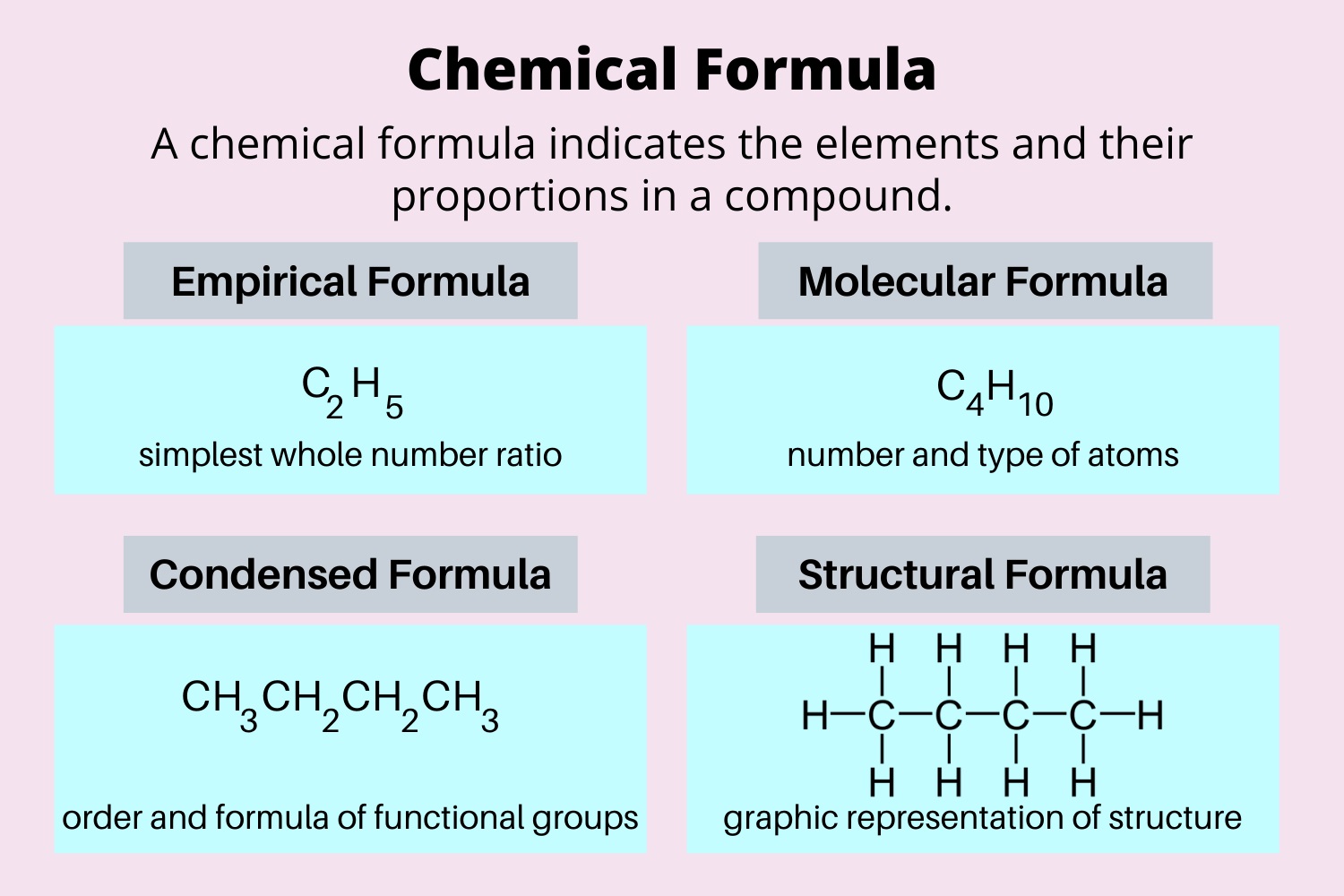
Chemical formulas can be empirical or molecular
Empirical formulas provide the simplest whole-number ratio of elements in a compound, while molecular formulas give the exact number of each element present in a molecule.
Chemical formulas help predict chemical reactions
Chemical formulas are crucial in understanding and predicting the outcome of chemical reactions, as they reveal the identities and quantities of reactants and products involved.
Chemical formulas can represent ionic compounds
In addition to molecular compounds, chemical formulas are used to represent ionic compounds, which consist of positively and negatively charged ions.
Chemical formulas are used in stoichiometry
Stoichiometry, a fundamental concept in chemistry, relies on the use of chemical formulas to determine the ratios of substances involved in a chemical reaction.
Chemical formulas are standardized
The symbols used in chemical formulas follow a standardized set of rules established by the International Union of Pure and Applied Chemistry (IUPAC) to ensure clear and consistent representation.
Chemical formulas can be written in different ways
Chemical formulas can be written as an empirical formula, molecular formula, or structural formula, each providing different levels of detail about the compound’s composition and structure.
Chemical formulas can include brackets and parentheses
Brackets and parentheses are used in chemical formulas to indicate the presence of polyatomic ions or complex groups within a compound.
Chemical formulas are crucial for balancing equations
When balancing chemical equations, chemical formulas play a vital role in ensuring that the law of conservation of mass is upheld, with the same number of atoms on both sides of the equation.
Chemical formulas can represent isotopes
Isotopes, which are atoms of the same element with different numbers of neutrons, can be denoted in chemical formulas by including the atomic mass as a superscript.
Chemical formulas can be used to calculate molar mass
By summing up the atomic masses of all atoms in a chemical formula, scientists can determine the molar mass of a compound, which is crucial for various calculations in chemistry.
Chemical formulas are used to name compounds
The systematic naming of compounds, known as chemical nomenclature, relies on chemical formulas to provide the necessary information for assigning names to different substances.
Chemical formulas can indicate the state of matter
In some cases, chemical formulas include symbols or letters to indicate the state of matter of a substance, such as (s) for solid, (l) for liquid, (g) for gas, or (aq) for aqueous solution.
Chemical formulas are essential for laboratory safety
Chemical formulas help scientists and lab technicians identify and handle different substances safely, ensuring proper storage, handling, and disposal procedures.
Chemical formulas can be used for quantitative analysis
Quantitative analysis in chemistry, such as determining the concentration of a solution or the mass of a compound, relies on the use of chemical formulas and stoichiometric calculations.
Chemical formulas can represent organic compounds
Organic compounds, which are compounds containing carbon, are represented using chemical formulas that reflect the unique bonding patterns and structures found in these substances.
Chemical formulas are constantly evolving
As new compounds are discovered and our understanding of chemistry deepens, the realm of chemical formulas continues to expand, evolving and adapting to accommodate new knowledge.
Conclusion
In conclusion, the world of chemical formulas is a fascinating and complex one. From the simple representations of elements to the intricate structures of compounds, chemical formulas provide us with a way to understand and interpret the building blocks of matter. They allow scientists to communicate ideas and findings, and they play a crucial role in many areas of chemistry and beyond.Exploring the countless chemical formulas out there, we discover incredible facts that highlight the diversity and ingenuity of the natural world. So, whether you’re a chemistry enthusiast or simply curious about the wonders of science, delving into the world of chemical formulas will surely leave you amazed and inspired.Remember, learning about chemical formulas is like unlocking a secret language that reveals the hidden beauty and complexity of the universe. So, keep exploring, keep discovering, and keep being intrigued by the incredible world of chemical formulas.
FAQs
Q: What is a chemical formula?
A: A chemical formula is a shorthand notation that represents the number and types of atoms or ions in a compound or molecule. It provides information about the elemental composition and arrangement of atoms in a substance.
Q: How are chemical formulas written?
A: Chemical formulas are written using symbols for the elements, along with numerical subscripts to indicate the number of atoms of each element present. For example, the chemical formula for water is H2O, indicating that it consists of two hydrogen atoms and one oxygen atom.
Q: What is the significance of chemical formulas?
A: Chemical formulas are of great significance in the field of chemistry as they help to identify and differentiate between different compounds. They also provide crucial information about the stoichiometry, polarity, and bonding in a substance.
Q: Can a chemical formula change?
A: Chemical formulas represent the fixed composition of a compound, and they do not change under normal circumstances. However, chemical reactions can occur where substances can combine, rearrange, or break apart, resulting in the formation of new compounds with different chemical formulas.
Q: Are there any exceptions or special cases in chemical formulas?
A: Yes, there are certain exceptions or special cases when it comes to chemical formulas. For example, some compounds, such as polymers, have variable compositions and their formulas are not fixed. Additionally, some elements, like hydrogen and oxygen, exist as diatomic molecules (H2 and O2) in their natural state.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
