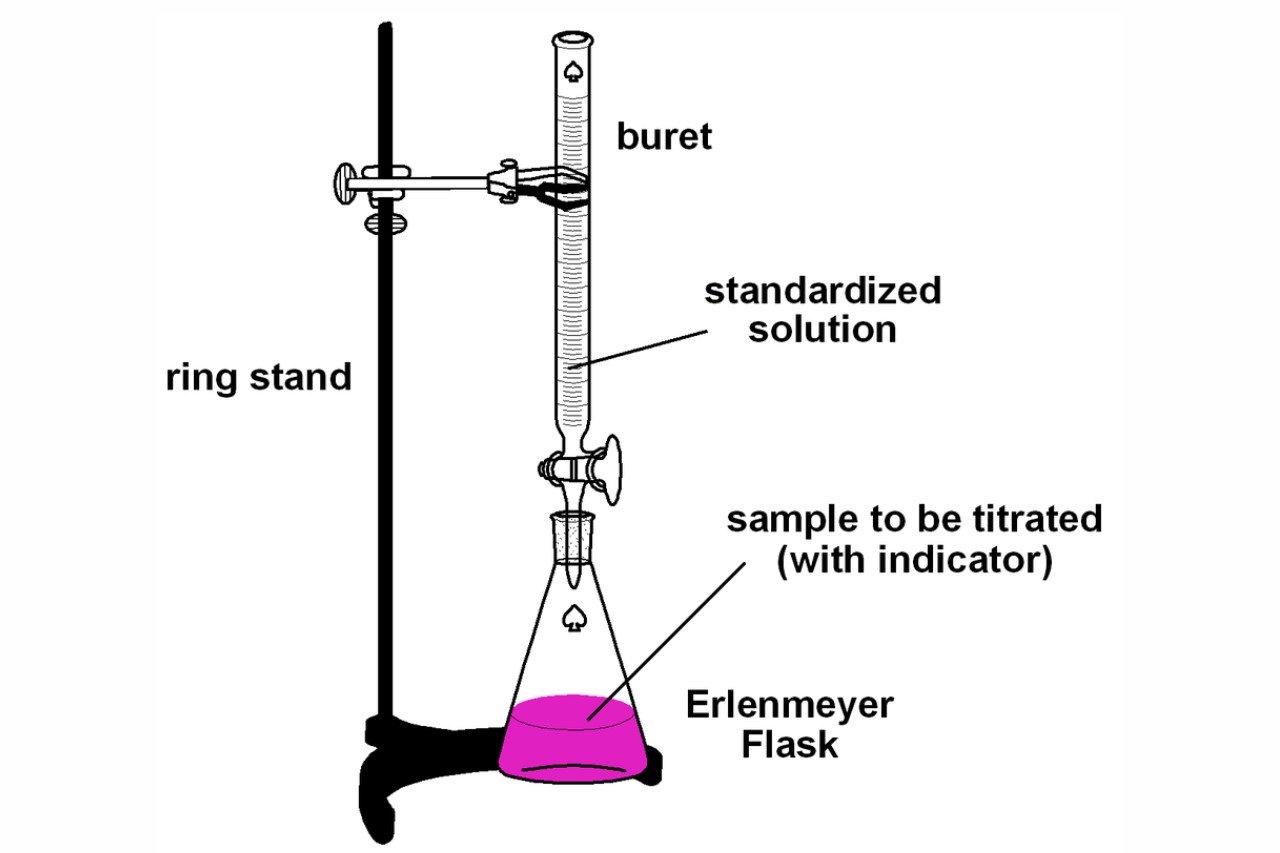
Acid-base titration is a fundamental concept in the field of chemistry that involves the quantitative analysis of acids and bases. It is a technique used to determine the concentration of an acid or a base solution by reacting it with a solution of known concentration. This process allows scientists to precisely measure the amount of acid or base present in a given sample.
In this article, we will delve into the fascinating world of acid-base titration and explore eight captivating facts that will deepen your understanding of this essential technique. From the history of titration to the different types of indicators used, we will uncover intriguing insights about this analytical method.
Key Takeaways:
- Acid-base titration is a cool chemistry technique that helps determine the acidity of substances and is used in making medicines and protecting the environment.
- It’s like a chemical detective game that scientists use to figure out the pH levels of water and the strength of medicines. It’s been around for a long time and is super important in chemistry!
The concept of acid-base titration dates back to the late 18th century.
Swedish chemist, Carl Wilhelm Scheele, is credited with laying the groundwork for acid-base titration in His discoveries paved the way for the development of this crucial analytical technique.
Acid-base titration can be used to determine the pH of a solution.
By using an appropriate indicator or pH meter, the endpoint of the titration can be determined, allowing for the calculation of the pH of the analyte solution, providing valuable information about its acidity or basicity.
Acid-base titration is commonly used in the pharmaceutical industry.
Pharmaceutical companies rely on acid-base titration to ensure the quality and potency of their products. It is used in the analysis of drugs, determining their acid or base content, and verifying their concentration.
Acid-base titration can be used to perform a neutralization reaction.
When an acid and a base react in a 1:1 ratio, they undergo a neutralization reaction. Acid-base titration allows for the determination of the exact ratio required to achieve neutralization.
Titration curves are graphical representations of acid-base titration data.
Titration curves show the pH of the solution being titrated plotted against the volume of the titrant added. These curves provide valuable information about the nature of the acid or base being analyzed.
Acid-base titration can be used to determine the molar mass of an unknown substance.
Using the known concentration of the titrant and the volume required for neutralization, the molar mass of the analyte can be calculated. This is especially useful when working with non-volatile or thermally unstable substances.
Acid-base titration can be performed using different indicators.
Indicators such as phenolphthalein and bromothymol blue can be used to determine the endpoint of the titration. These indicators change color when the solution reaches a specific pH, helping to visually identify when neutralization has occurred.
Acid-base titration is an essential technique in environmental analysis.
Environmental scientists use acid-base titration to measure the acidity of natural water bodies. This technique helps monitor the environmental impact of pollution and assess the efficiency of water treatment processes.
These 8 captivating facts about acid-base titration demonstrate the versatility and importance of this analytical technique in various scientific disciplines. From determining pH levels to assessing the quality of pharmaceutical products, acid-base titration plays a vital role in advancing our understanding of chemical substances.
Conclusion
Acid-base titration is a fascinating field of study that plays a crucial role in various scientific disciplines, especially chemistry. By understanding the principles behind acid-base titration, scientists and researchers can make accurate measurements and obtain valuable data about the chemical reactions taking place.Through this article, we have explored eight captivating facts about acid-base titration. We learned about the concept of titration and how it is used to determine the concentration of an unknown solution. We delved into the importance of indicators and their role in indicating the end point of a titration. We also discovered the different types of titrations, such as strong acid-strong base and weak acid-strong base titrations.Furthermore, we explored the significance of titration curves, which provide a graphical representation of the pH changes during a titration process. We also discussed the concept of equivalence point and how it relates to the stoichiometry of the reaction.Overall, acid-base titration is an essential technique that allows scientists to measure and understand the interactions between acids and bases. It empowers researchers to determine the concentration of unknown solutions and aids in various analytical applications. By delving deeper into the world of acid-base titration, we gain a deeper appreciation for its practical applications and its impact on scientific advancements.
FAQs
1. What is acid-base titration?
Acid-base titration is a laboratory technique used to determine the concentration of an unknown acid or base solution by reacting it with a known solution of acid or base.
2. How is the end point of a titration determined?
The end point of a titration is determined using an indicator, which changes color when the equivalence point is reached and signals the completion of the reaction.
3. What types of titrations exist?
There are different types of titrations, including strong acid-strong base, weak acid-strong base, and weak acid-weak base titrations.
4. What is a titration curve?
A titration curve is a graphical representation of the pH changes during a titration process. It helps determine the equivalence point and gives insights into the stoichiometry of the reaction.
5. What is the equivalence point?
The equivalence point is the point in a titration where the moles of the acid equal the moles of the base. It represents the completion of the reaction.
6. What are some practical applications of acid-base titration?
Acid-base titration has numerous practical applications, including pharmaceutical analysis, environmental testing, and quality control in industries such as food and beverage.
7. Can titration be performed without an indicator?
Yes, titration can be performed without an indicator using pH meters or potentiometric titration, which relies on measuring the change in potential during the titration process.
8. Why is acid-base titration important in chemistry?
Acid-base titration is important in chemistry because it allows scientists to accurately determine the concentration of unknown solutions, study the properties of acids and bases, and understand the stoichiometry of chemical reactions.
Acid-base titration is a powerful technique used in various fields, from pharmaceuticals to environmental analysis. Understanding titration curves and their graphical representations can provide deeper insights into the fascinating world of chemistry. Mastering the concepts behind acid-base titration opens doors to new possibilities in research and industry. Keep exploring this captivating subject to unravel more of its secrets and potential applications.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
